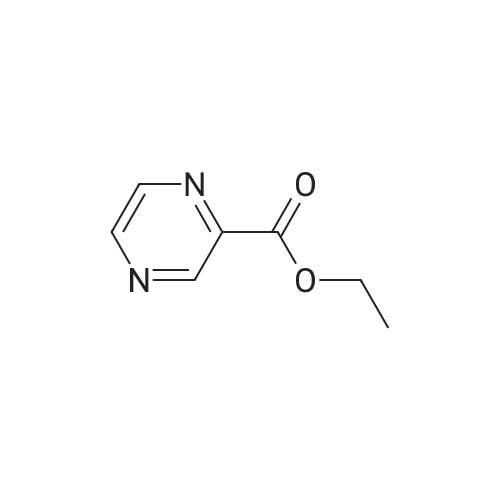
| 46% |
With bromine; sodium nitrite; In chloroform; water; hydrogen bromide; |
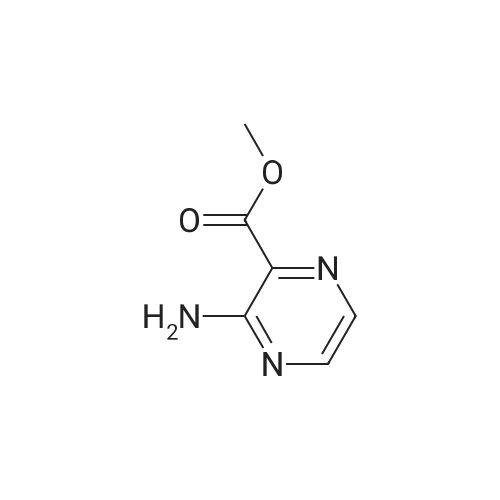
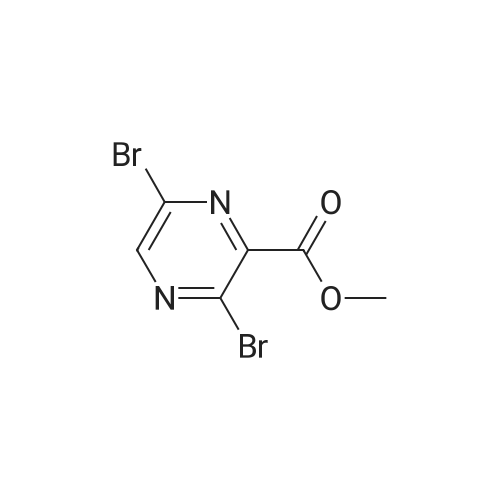
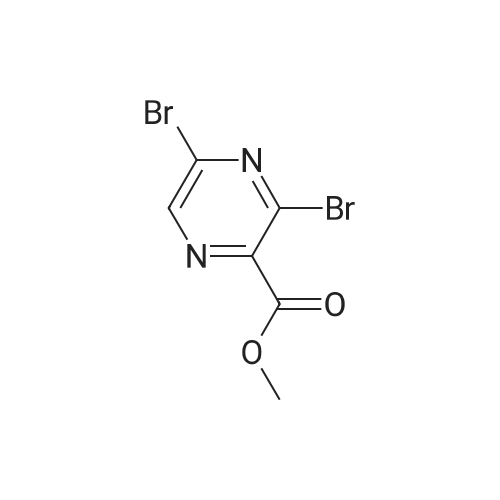
EXAMPLE 157A Methyl 3-bromopyrazine-2-carboxylate To a rapidly stirring heterogeneous mixture of 3-aminopyrazine-2-carboxylic acid methyl ester (2.00 g, 13.1 mmol) in 48% hydrobromic acid (7.9 mL) cooled to 0 C. was added bromine (2.00 mL, 6.2 g, 38.8 mmol) dropwise over 5 minutes. Then a solution of sodium nitrite (2.27 g, 32.8 mmol) in 9.5 mL of water was added dropwise over 10 minutes. The reaction mixture was stirred at 0 C. for 15-30 minutes and then basified with 60 mL of saturated sodium bicarbonate solution and extracted with ethyl acetate followed by chloroform. The combined organic extracts were dried over magnesium sulfate and concentrated under reduced pressure. The residue obtained was flash chromatographed on silica gel eluding with mixtures of hexane and ethyl acetate to afford the title compound (1.265 g, 46%). m.p. 43.5-44 C. 1 H NMR (CDCl3, 300 MHz) delta 4.04 (s, 3H), 8.50 (bs, 1H), 8.60 (bs, 1H). MS (DCl/NH3) m/e 217/219 (M+H)+, 234/236 (M+H+NH3)+. |
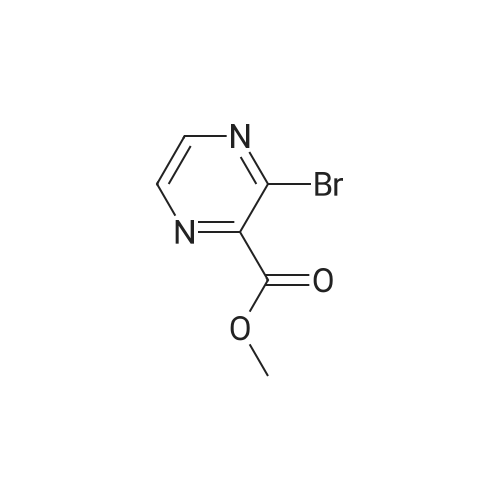
| 12% |
With tert.-butylnitrite; copper(I) bromide; In acetonitrile; at 60℃; for 0.25h; |
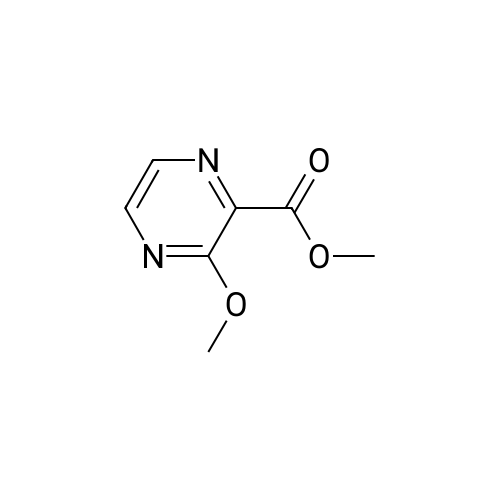
Example 81; Synthesis of (3,5-bis-trifluoromethyl-benzyl)- [3-(cycIopentylmethyl-ethyl-amino)- pyrazin-2-ylmethyl]-carbamic acid methyl ester; Step (i): Synthesis of 3-bromo-pyrazine-2-carboxylic acid methyl ester; Copper bromide (1.36 g, 6.1 mmol) and £-butyl nitrite (0.78 g, 7.6 mmol) were added to a 50 mL round bottom flask along with acetonitrile (2 niL), and this mixture was heated at 60 0C for 5 min. After this time, 3-amino-pyrazine-2-carboxylic acid methyl ester (0.8 g, 5.09 mmol) was added portion- wise, with stirring, and stirring was continued at the same temperature for another 10 min. The reaction mixture was then cooled to RT, poured into 100 mL of dilute HCL (2N), and then extracted with diethyl ether (3 x 50 mL). The combined organic layer was washed with dilute HCl, dried over sodium sulfate, and then concentrated under vacuum to afford the title compound (0.139 g), yield: 12 %.1H NMR (CDCl3, 400 MHz): d 8.58 (m, 2H), 4.04 (s, 3H)) ; m/z (CI-MS) 217 (M+) ; IR (neat, cm-1): 3385, 2955, 1742 |
|
With hydrogen bromide; bromine; sodium nitrite; In water; |
(A) Methyl 2-bromo-3-pyrazine carboxylate To a stirred mixture of 12.7 g. of methyl 2-amino pyrazine carboxylate and 47 ml. of 48% hydrobromic acid there was added, dropwise, 12.6 ml. of bromine keeping the temperature at 0. A solution of 14.4 g. of sodium nitrite in 60 ml. of water was then added, dropwise, at 0 and the reaction mixture stirred for 15 minutes. The reaction mixture was basified to pH 8 with sodium bicarbonate and extracted with ethyl acetate and again with chloroform. The organic layers were dried over magnesium sulfate, filtered and concentrated to a yellow oil. Recrystallization from ether-hexane yielded the product, m.p. 43-45 C. |
|
With hydrogen bromide; bromine; sodium nitrite; In water; |
(A) Methyl 2-bromo-3-pyrazine carboxylate: To a stirred mixture of 12.7 g. of methyl 2-amino pyrazine carboxylate and 47 ml. of 48% hydrobromic acid there was added, dropwise, 12.6 ml. of bromine keeping the temperature at 0. A solution of 14.4 g. of sodium nitrite in 60 ml. of water was then added, dropwise, at 0 and the reaction mixture stirred for 15 minutes. The reaction mixture was basified to pH 8 with sodium bicarbonate and extracted with ethyl acetate and again with chloroform. The organic layers were dried over magnesium sulfate, filtered and concentrated to a yellow oil. Recrystallization from ether-hexane yielded the product, m.p. 43-45 C. |
|
With hydrogen bromide; bromine; sodium nitrite; In water; |
(A) Methyl 2-bromo-3-pyrazine carboxylate To a stirred mixture of 12.7 g. of methyl 2-amino pyrazine 3-carboxylate and 47 ml. of 48% hydrobromic acid there is added, dropwise, 12.6 ml. of bromine keeping the temperature at 0. A solution of 14.4 g. of sodium nitrite in 60 ml. of water is then added, dropwise, at 0 and the reaction mixture stirred for 15 minutes. The reaction mixture is basified to pH 8 with sodium bicarbonate and extracted with ethyl acetate and again with chloroform. The organic layers are dried over magnesium sulfate, filtered and concentrated to a yellow oil. Recrystallization from ether-hexane yields the product, m.p. 43-45 C. |
|
With hydrogen bromide; bromine; sodium nitrite; In water; |
(A) Methyl 2-bromo-3-pyrazine carboxylate: To a stirred mixture of 12.7 g. of methyl 2-aminopyrazine 3-carboxylate and 47 ml. of 48% hydrobromic acid there is added, dropwise, 12.6 ml. of bromine keeping the temperature at 0. A solution of 14.4 g. of sodium nitrite in 60 ml. of water is then added, dropwise, at 0 and the reaction mixture stirred for 15 minutes. The reaction mixture is basified to pH 8 with sodium bicarbonate and extracted with ethyl acetate and again with chloroform. The organic layers are dried over magnesium sulfate, filtered and concentrated to a yellow oil. Recrystallization from ether-hexane yields the product, m.p. 43-45 C. |
|
With hydrogen bromide; bromine; sodium nitrite; In water; at 0℃; for 2h; |
Step 1: synthesis of methyl 3-bromopyrazine-2-carboxylate (99)To a solution of methyl 3-amino-2-pyrazinecarboxylate (13.06 mmol, 2 g) in hydrobromic acid (13.06 mmol, 7.4 ml, 1.057 g) at 0C was added bromine (38.9 mmol, 2 mL, 6.22 g) drop wise and then a solution of sodium nitrite (33.3 mmol, 2.3 g) in water (4 mL). The reaction was stirred for 2h at 0C and the reaction mixture was extracted with CH2CI2. Organic layer was dried and evaporated to give crude methyl 3- bromopyrazine-2-carboxylate 101 (1.2gr, 43%). (m/z) = 217 and 219 (M+H)+. |
|
With hydrogen bromide; bromine; sodium nitrite; In water; at 0℃; for 2h; |
Step 1: synthesis of methyl 3-bromopyrazine-2-carboxylate (99)[0305]To a solution of methyl 3-amino-2-pyrazinecarboxylate (13.06 mmol, 2 g) in hydrobromic acid (13.06 mmol, 7.4 ml, 1.057 g) at 0 C. was added bromine (38.9 mmol, 2 mL, 6.22 g) drop wise and then a solution of sodium nitrite (33.3 mmol, 2.3 g) in water (4 mL). The reaction was stirred for 2 h at 0 C. and the reaction mixture was extracted with CH2Cl2. Organic layer was dried and evaporated to give crude methyl 3-bromopyrazine-2-carboxylate 101 (1.2 gr, 43%). (m/z)=217 and 219 (M+H)+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping