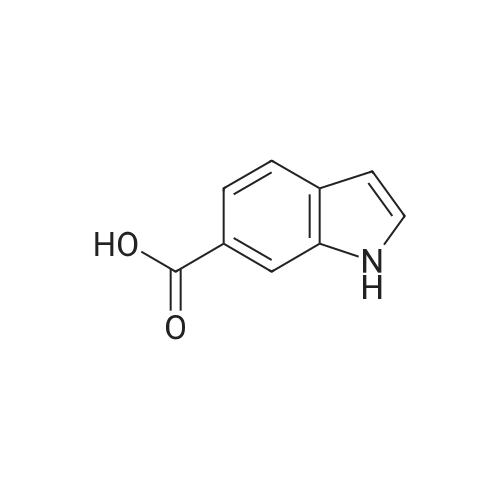
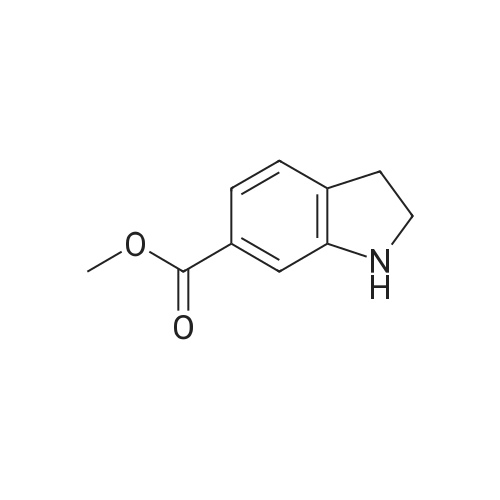
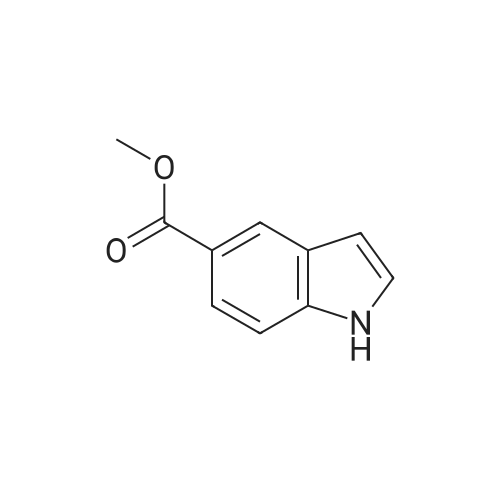
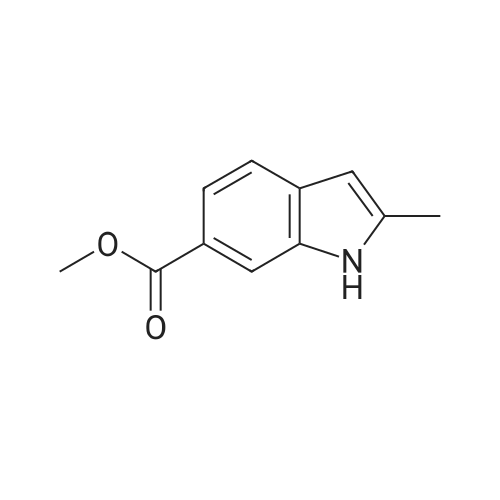
| 75% |
With sodium cyanoborohydride; acetic acid; In dichloromethane; at 0 - 25℃; |
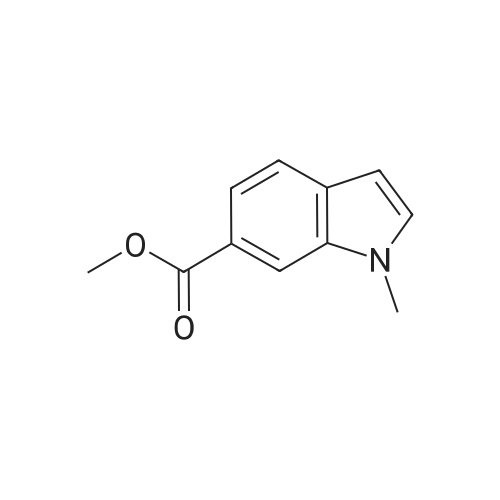
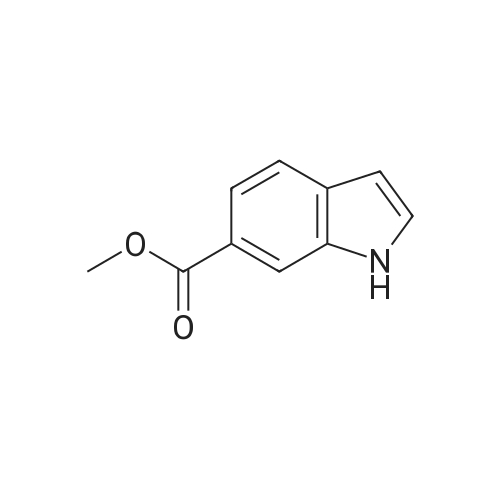
NaCNBH3 (11.49 g; 0.04 equiv.) was added at 0° C., over a period of 10 minutes, to a solution of methyl 1H-indole-6-carboxylate (8 g; 1 equiv.) in acetic acid (80 ml). The reaction mixture was stirred at 0° C. for 20 minutes and then warmed to room temperature and stirred for 1 hour at room temperature. When the conversion was complete, the acetic acid was distilled off under reduced pressure and the resulting residue was dissolved in dichloromethane. The resulting phases were separated. The organic phase was washed with 1N sodium hydroxide solution and dried over sodium sulfate. After removal of the solvent under reduced pressure, purification was carried out by column chromatography (silica gel, 10-15percent ethyl acetate/hexane). Methyl indoline-6-carboxylate (6 g; 75percent) was obtained in the form of a solid. |
| 73% |
With sodium cyanoborohydride; acetic acid; at 15℃; for 5h; |
A solution of methyl lH-indole-6-carboxylate ( 4.80 g, 27.4 mmol ) in acetic acid ( 40 mL ) was cooled to 15 0 C and then treated with sodium cyanoborohydride ( 6.90 g, 0.11 mmol ) added in small portions over 30 min. After 5 h at 15 ° C, the reaction mixture was diluted with a mixture of ice and water ( 200 mL) and carefully adjusted to pH 9 -10 with solid potassium carbonate. The aqueous phase was extracted three times with dichloromethane and the combined organic phase was washed with brine and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure and the residual oil was chromatographed on silica gel ( elution toluene - ethyl acetate 8 : 2 ) to give 3.54 g ( 73 percent yield ) of the title material as yellowish solid. HPLC (Method A): 0.9 min ( tailing ). HRMS (ESI) calcd for Ci0H12NO2 [M+H]+ m/z 178.0863, found 178.0882. 1H NMR (CDC13, 400 MHz) delta ppm: 7.40 (dd, J = 7.6, 1.5 Hz, 1H), 7.25 (d, J = 1.5 Hz, 1H), 7.12 (br. d, J = 7.6 Hz, 1H), 3.85 (s, 3H), 3.59 (t, J = 8.5 Hz, 2H), 3.05 (t, J = 8.5 Hz, 2H). |
| 49% |
|
A stirred solution of 2A (1 g, 5.7 mmol) in DCM (20 ml_) and TFA (10 mL) at -200C was treated with Et3SiH (10 mL). The reaction was warmed to RT slowly and stirred thereafter for 17 h. The reaction was quenched with 2 N NaOH until pH 8. The mixture was extracted with DCM (100 mL x 3). The combined organic layer was dried (Na2SO4), filtered and concentrated under reduced pressure. Chromatography (20percent EtOAc/hexanes) provided 2B (0.5g, 49percent). |
| 24% |
With sodium cyanoborohydride; acetic acid; at 30℃; for 16h; |
To a solution of methyl IH-indole-6-carboxylate (5.0 g, 28.54 mmol) in AcOH (30 mL) wasadded NaBH3CN (5.4 g, 85.62 mmol). The mixture was stirred at 30 °C for 16 h. The reactionwas quenched with sat. aq. NaHCO3 (300 mL) and extracted with EtOAc (100 mL x 3). The combined organic layers were washed with brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The crude residue was purified by silica gel chromatography (petroleum ether / EtOAc = 50 : I to 3 : I) to give the title compound (1.2 g, 24percent) as a whitesolid. ?H NMR (400 MHz, DMSO-d6) oe 7.16 (d, J7.6 Hz, IH), 7.14 (d, J=7.6 Hz, IH), 5.75 (s, IH), 3.78 (s, 3H), 3.52 (t,J 8.4 Hz, 2H), 2.95 (t,J 8.4 Hz, 2H). |
|
With sodium cyanoborohydride; acetic acid; at 0 - 15℃; for 2h; |
Dissolve methyl indole-6-carboxylate (5.00 g, 28.5 mmol) in acetic acid (50 mL)In a 100 mL single-mouth bottle, sodium cyanoborohydride (8.85 g, 142 mmol) was slowly added at 0 °C.After completion, the reaction was carried out at 15 ° C for 2 hours. After the reaction is complete,The reaction solution was dried to dryness and then purified and evaporated with methylene chloride (200 mL).Extracted with dichloromethane (150 mL × 3),The combined organic phases were dried and concentrated to give the title compound. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping