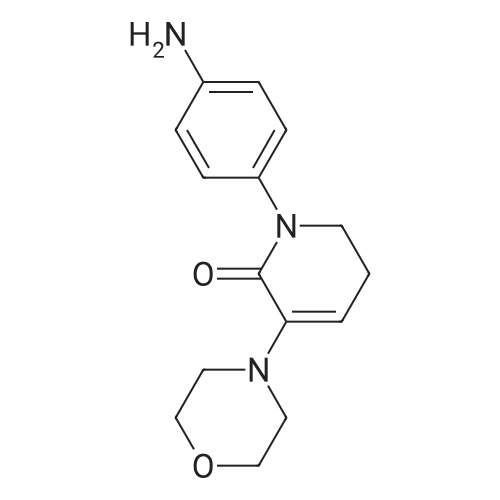
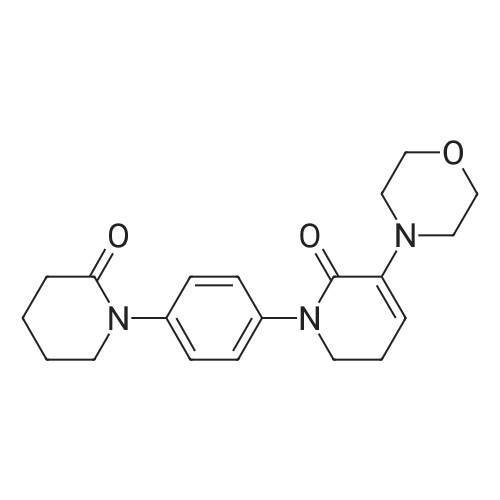
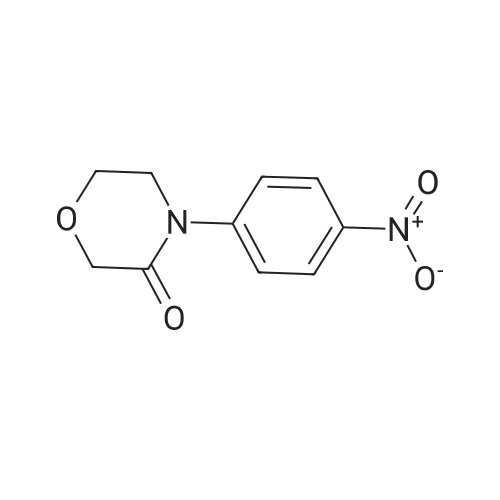
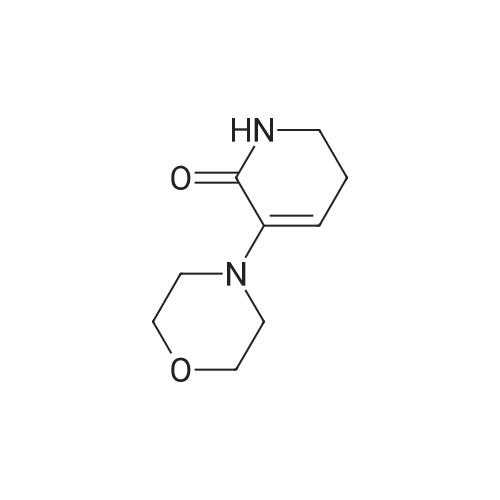
| 93.2% |
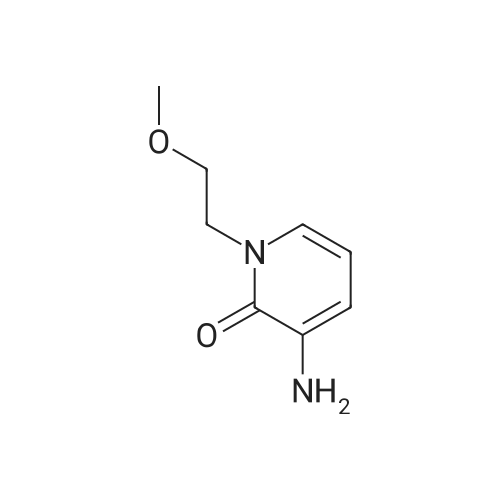
With sodium disulfide; water; In ethanol; at 50 - 55℃; for 3h;Large scale; |
2000L reactor by adding purified water 570kg,Stirring under the addition of sodium hexahydrate 569.7kg,Heating to 50-55 C, stirring about 30 minutes to dissolve, add sulfur 76kg, stirring and stirring for 1 hour to dissolve, prepared to obtain sodium disulfide.Followed by adding ethanol 570kg,90 kg of the compound of formula II-1,50-55 C Insulation reaction 3h,The reaction was complete and extracted with 900 kg of dichloromethane.The organic layer was washed with 500 kg of saturated brine and dried over 50 kg of anhydrous sodium sulfate for 2 hours. The dichloromethane was distilled off under reduced pressure to give 75.6 kg of the 1-1 compound, the yield was 93.2%, and the HPLC purity was 100%.As shown in Figure 1, almost no impurities in the product produced, the raw material reaction is complete. |
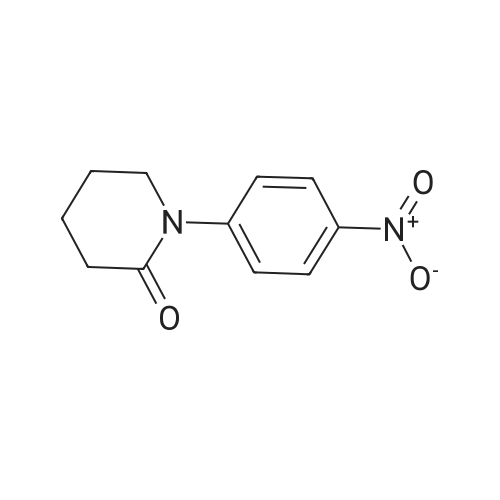
| 91% |
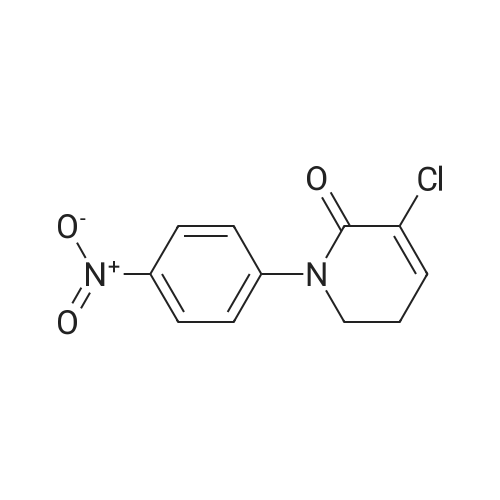
With sodiumsulfide nonahydrate; In ethanol; at 60℃; for 1h; |
Compound 6 (10g, 0.033mol), NaS.9H2O (32g, 0.13mol), Anhydrous ethanol 200mol added to the reaction bottle, adding reaction bottle, 60 C reaction 1h, The ethanol was evaporated and the product was worked up as a pale yellow solid (8.3g, 0.03mol) in a yield of 91% |
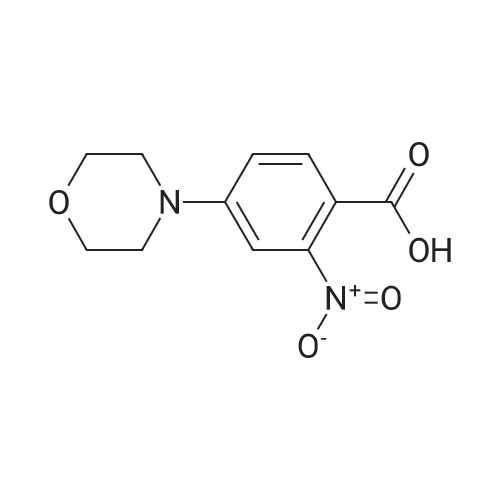
| 91% |
With sodiumsulfide nonahydrate; In methanol; water; at 40 - 45℃; for 2.75h; |
A solution of Sodium sulfate nonahydrate (197 g, 2.5 eq) in water (350 ml_) was dosed over 45 minutes at T=40/45C into a mixture of compound of formula (XI) (100 g,1.0 eq.) and methanol (1000 mL). The mixture was stirred at T=40/45C for additional 2 hours to reach reaction completion. The mixture was then distilled at reduced pressure and Tmax=45C until 930 ml_ of solvent were removed. The resulting slurry was cooled down to T=20/25C, kept stirring for 1 hour at this temperature and then filtered washing the wet cake with water (2 x 100 mL). Upon drying at reduced pressure and T=65C for 10 hours, 82 g of the compound of formula (X) were obtained. (91 % yield, HPLC A%: compound of formula (X) 96.16%, Said compound of formula (X) thus obtained contained the following dimer impurities: - 0,66% (HPLC A/A%) dimer impurity of formula (VI), - 1 ,50% (HPLC A/A%) dimer impurity of formula (VII). |
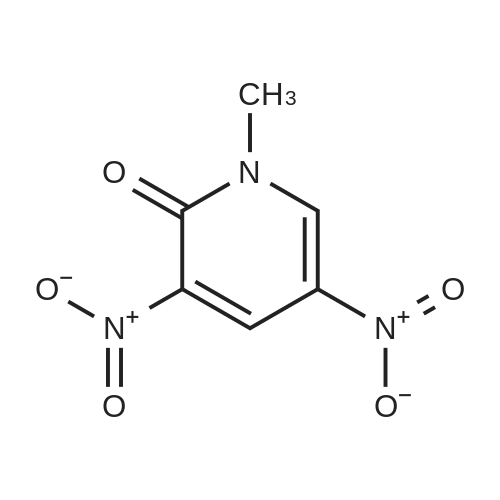
| 74.1% |
With sodium sulfide; water; In ethanol; at 50℃;Inert atmosphere; |
The 1d (18.0g, 59.3mmol) was dissolved in ethanol (180ml) was added sodium sulfide nonahydrate (28.4g, 118.6mmol), was added water (60ml) to give a reaction mixture, and the mixture was reacted at 50 C .The reaction was refluxed overnight. Tracking progress of the reaction by TLC, the reaction was completed, the ethanol under reduced pressure, extracted three times with ethyl acetate, the combined organic phases concentrated to remove, dried over anhydrous magnesium sulfate, filtered, and the solvent removed by distillation under reduced pressure to give 1e (12.0g, yellow solid) yield: 74.1% |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping