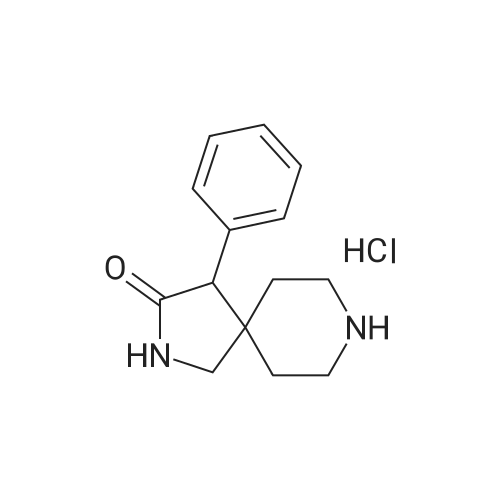
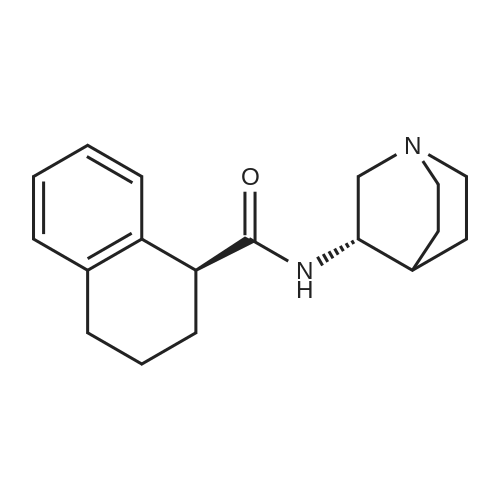
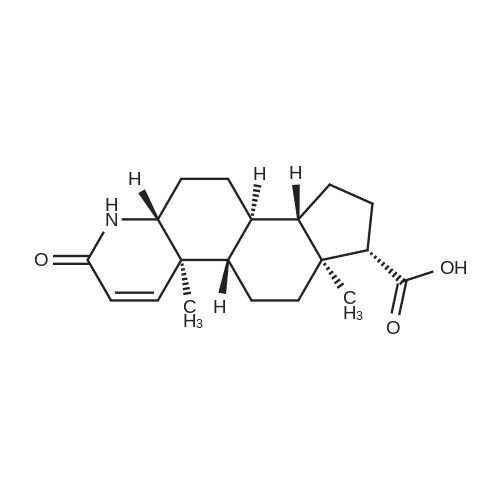
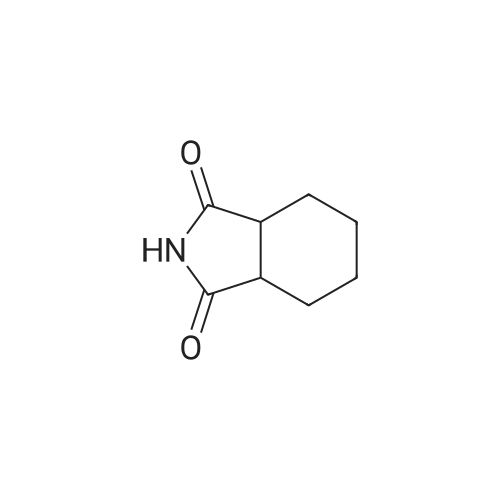
| 88% |
Stage #1: With methanesulfonic acid In tetrahydrofuran; water at 34 - 44℃; for 2.75 h; Heating / reflux
Stage #2: With lithium aluminium tetrahydride In tetrahydrofuran for 18.5 h; Heating / reflux |
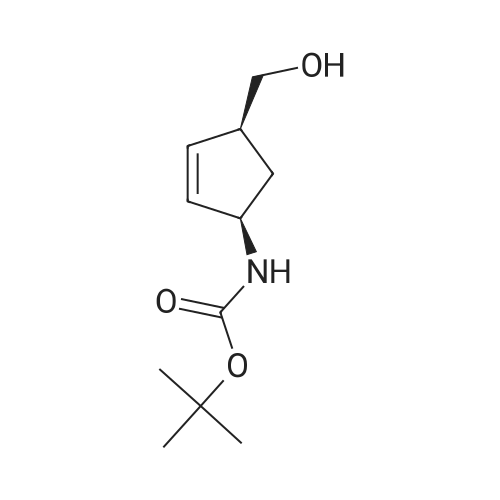
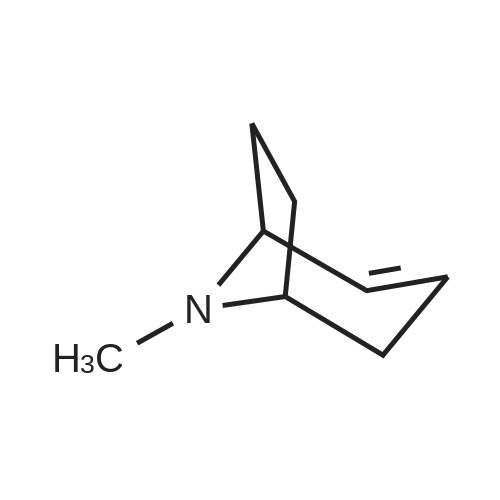
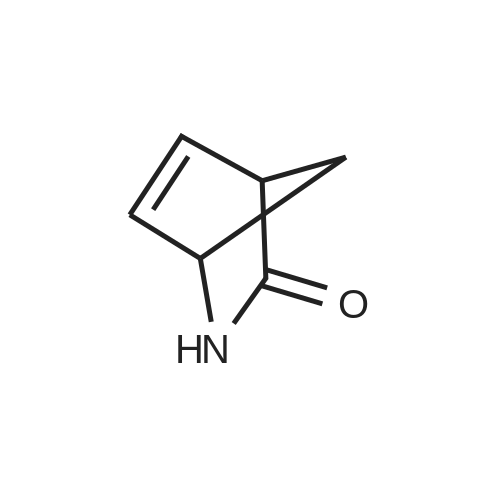
The first filtrate of Example 2 containing (-)-(1S, 4R-amino-2-cycopentene-1-methanol was cooled in anice-acetone bath and treated with di-tert-butyl dicarbonate (199.42 g, 0.9265 mol, Aldrich). The mixture was concentrated under vacuum to a volume of 300 mL, and added to the second filtrate of Example 2 that had meanwhile been cooled in an ice-acetone bath. The mixture was allowed to stir and warm to room temperature over the course of 18 hours, during which time gas evolved and a clear solution formed. This solution was combined with the last filtrate of Example 2 which had been evaporated under vacuum to a mixture of oil and solids. The resulting solution was evaporated under vacuum to an oil. The oil was partitioned between ethyl acetate (300 mL) and phosphate buffer (100 mL of 1.5 molar potassium dihydrogen phosphate adjusted to pH 7.0 with 50percent sodium hydlroxide-water). The phases were separated, the aqueous phase was reextracted twice with ethyl acetate (200 mL). The organic phases were dried over sodium sulfate and filtered through silica gel (50 g.). The solvent was removed under vacuum to give an oil (220.78 g), which was taken up in hexanes (300 mL). A minimum amount of ethyl acetate (about 50 mL) was added in order to dissolve the oil, and the solution was set to crystallize over the course of three days. The crystals were filtered off, washed with 20percent ethyl acetate/hexanes, and dried by suction to a constantweight (156.1 g, 0.732 mol, 82.6percent of theory) of the title compound; m.p. 73-73.7° C.; 1 H-NMR (DMSO-d6) 5: 6.72 (d, J=7.9 Hz, 1H, NH), 5.80 and 5.60 (two m, 2H, CHCH), 4.59 (t, J=5.2 Hz, 1H, OH), 4.45 (m, 1H, CHN), 3.35 (m, overlapping H2O, CH2O), 2.60 (m, 1H, CH), 2.30 (m, 1H, 1/2 CH2), 1.40 (s, 9H, C(CH3)3), 1.2 (m, 1H, 1/2CH2); [α]20589-2.78°, [α]20578-2.84°, [α]20546-3.6°, [α]20436-3.39°, [α]20365-0.95° (c=5.07, methanol); Cl-MS (CH4) 214 (M+1); TLC (silica, 10percent methanol-chloroform, iodine visualization), Rf=0.51. Anal. Calcd. for C11H19O13N: C, 61.95; H, 8.98, N, 6.57. Found: C, 61.87; H, 8.96; N, 6.59. An additional 10.14 g of crystalline material was recovered from the mother liquor by crystallization and chromatography, bringing the total yield to 166.24 g (0.780 mol, 87.9percent of theory from the lactam starting material of Example 1). It was also found convenient to prepare the title compound directly from 2-azabicyclo [2.2.1] hept-S-en-3-one, either racemic or the (-) enantiomer, as follows. (-)2-Azabicyclo [2.2.1] hept-S-en-3-one (6.00 g, 55.0 mmol) in anhydroustetrahydrofuran (30 mL) was warmed to 34° C. and stirred while methanesulfonic acid (3.6 mL, 55 mmol) and water (0.99 mL, 55 mmol) were added dropwise over 10 minutes. An exotherm of 10° C. was observed within 5 minutes and a crystalline solid began to precipitate. The mixture was refluxed (oil bath at 74° C.) for 2.5 hours. The mixture was cooled to -10° C. and a solution of lithium aluminum hydride (1.0 M intetrahydrofuran, 100 mL) added. The first 15 mL was added over 10 minutes and an exotherm of 7° C. noted. The remaining 85 mL was added rapidly with no further exotherm noted. The mixture was brought to reflux over 30 minutes and reflux continued for 18hours. The mixture was cooled to 25° C.and sodium fluoride (25.2 g, 0.600 mole) was added and, after stirring for 30 minutes water (5.3 mL) was added dropwise over 10 minutes to the cooled (0° C.) mixture. The mixture was stirred for 30 minutes at 25° C. and di-tert-butyl dicarbonate (12.6 mL, 55.0 mmol) was added. This mixture was stirred for 16 hours, filtered, and the cake triturated with ethyl acetate (2.x.50 mL). The combined filteratewash was washed with water (20 mL), dried (Na2SO4), evaporated, and the residual syrup crystallized from ethyl acetate:hexanes/1:2 (30 mL) to give title compound as white crystals (10.32 g, 88percent), identical in properties to the above-described samp |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping