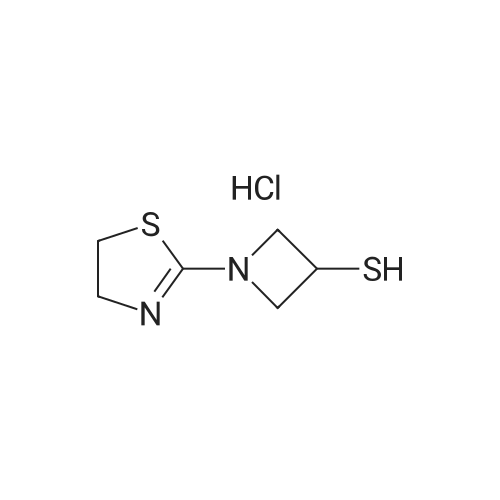
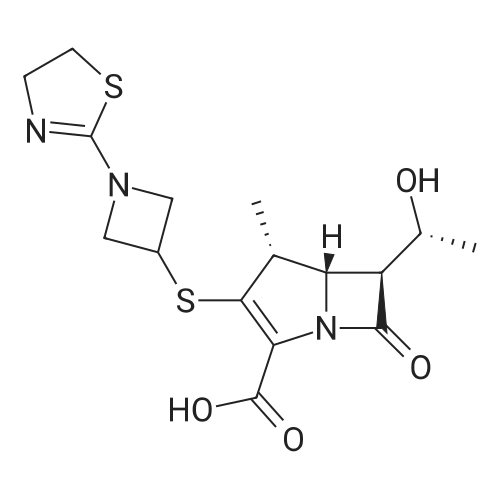
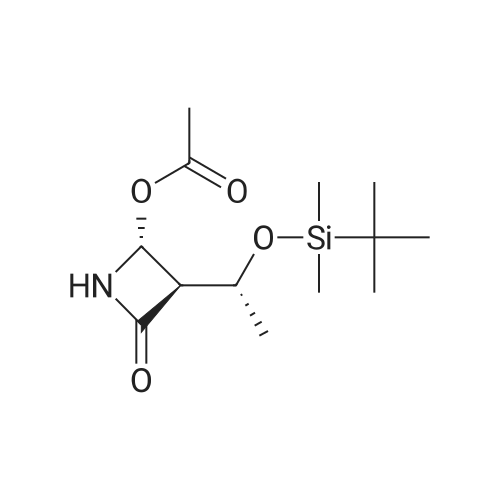
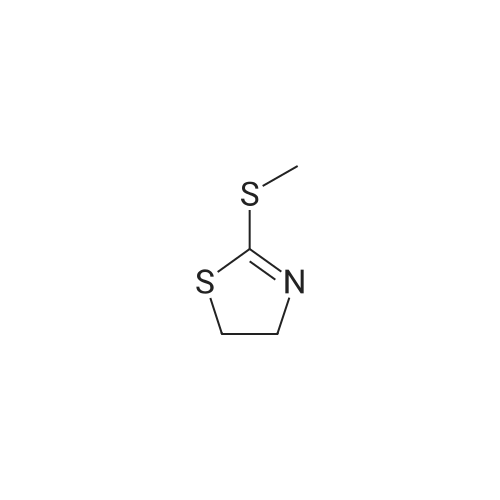
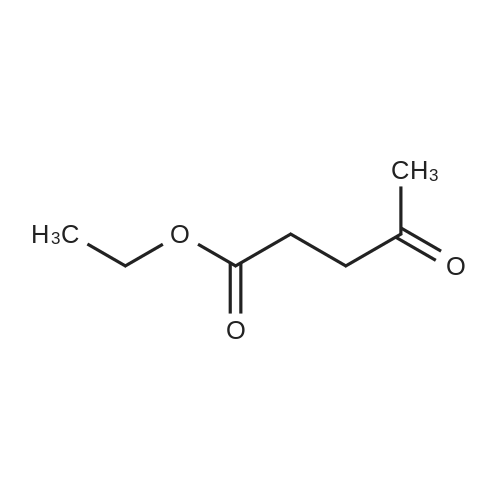
| 57% |
With polyphosphoric acid; at 120℃; for 2.0h; |
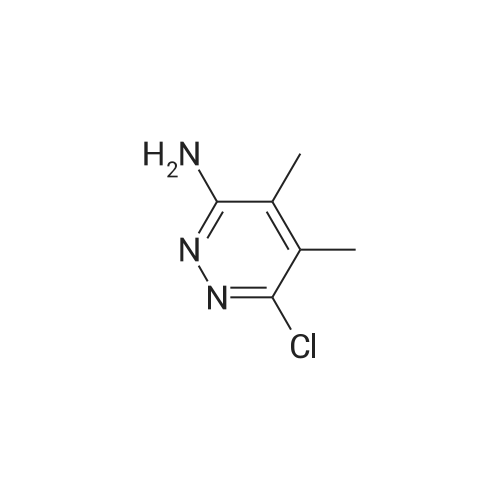
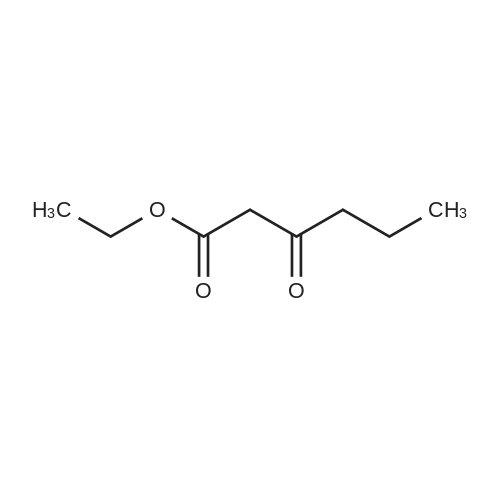
To a solution of 6-choro-3-amino-4,5-dimethylpyridazine (550 mg) in polyphosphoric acid (4 mL) was added ethyl 3-oxopentanoate {913 pL). The mixture was heated to 85 “C for 1 hour, then 120 C for 1 hour. While hot, the mixture was slowly transferred to a stirred solution of saturated aqueous NaHCC {~150 mL) and chloroform/IPA (4:1) (50 mL). Upon complete addition, the mixture was stirred for 10 minutes then the organic layer was isolated. The aqueous layers was further extracted with chloroform/IPA (4:1) (x3) and the organic layers were pooled, dried over MgS04, filtered, and concentrated under vacuum. Upon cooling, a solid precipitate was observed. Hexanes was added, the suspenion was sonicated, the solid was collected by vacuum filtration, and washed with hexanes to provide the title compound (469 mg, 57% yield) >95% dean by LCMS. NMR (400 MHz, CDC ) d 6.50 (s, 1H), 2.70 (q, J 7.6 Hz, 2H), 2.61 (s,3H), 2.48 (s, 3H), 1.30 (?., J = 7.6 Hz, 3H). ES-MS [M+l]+: 238. |
| 57% |
With polyphosphoric acid; at 120℃; for 2.0h; |
To a solution of 6-choro-3-amino-4,5-dimethylpyridazine (550 mg) in polyphosphoric acid (4 mL) was added ethyl 3-oxopentanoate {913 pL). The mixture was heated to 85 “C for 1 hour, then 120 C for 1 hour. While hot, the mixture was slowly transferred to a stirred solution of saturated aqueous NaHCC {~150 mL) and chloroform/IPA (4:1) (50 mL). Upon complete addition, the mixture was stirred for 10 minutes then the organic layer was isolated. The aqueous layers was further extracted with chloroform/IPA (4:1) (x3) and the organic layers were pooled, dried over MgS04, filtered, and concentrated under vacuum. Upon cooling, a solid precipitate was observed. Hexanes was added, the suspenion was sonicated, the solid was collected by vacuum filtration, and washed with hexanes to provide the title compound (469 mg, 57% yield) >95% dean by LCMS. NMR (400 MHz, CDC ) d 6.50 (s, 1H), 2.70 (q, J 7.6 Hz, 2H), 2.61 (s,3H), 2.48 (s, 3H), 1.30 (?., J = 7.6 Hz, 3H). ES-MS [M+l]+: 238. |
| 57% |
With polyphosphoric acid; at 120℃; for 2.0h; |
To a solution of 6-choro-3-amino-4,5-dimethylpyridazine (550 mg) in polyphosphoric acid (4 mL) was added ethyl 3-oxopentanoate {913 pL). The mixture was heated to 85 “C for 1 hour, then 120 C for 1 hour. While hot, the mixture was slowly transferred to a stirred solution of saturated aqueous NaHCC {~150 mL) and chloroform/IPA (4:1) (50 mL). Upon complete addition, the mixture was stirred for 10 minutes then the organic layer was isolated. The aqueous layers was further extracted with chloroform/IPA (4:1) (x3) and the organic layers were pooled, dried over MgS04, filtered, and concentrated under vacuum. Upon cooling, a solid precipitate was observed. Hexanes was added, the suspenion was sonicated, the solid was collected by vacuum filtration, and washed with hexanes to provide the title compound (469 mg, 57% yield) >95% dean by LCMS. NMR (400 MHz, CDC ) d 6.50 (s, 1H), 2.70 (q, J 7.6 Hz, 2H), 2.61 (s,3H), 2.48 (s, 3H), 1.30 (?., J = 7.6 Hz, 3H). ES-MS [M+l]+: 238. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













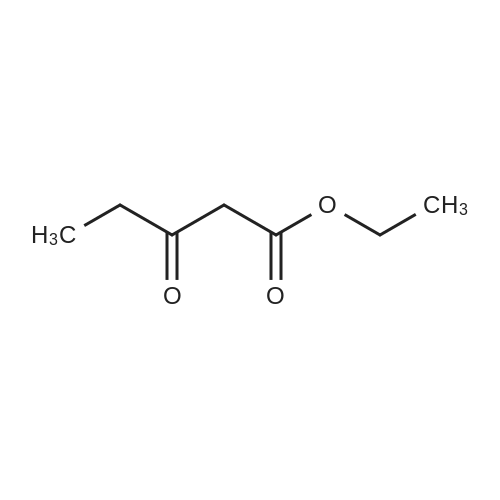

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping