| 86% |
With potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In dimethyl sulfoxide; at 20 - 80℃; for 3h; |
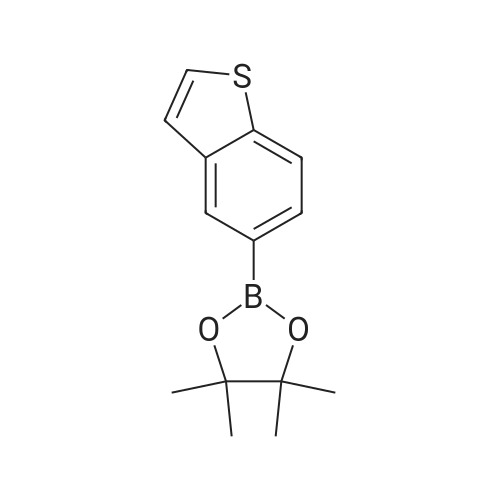
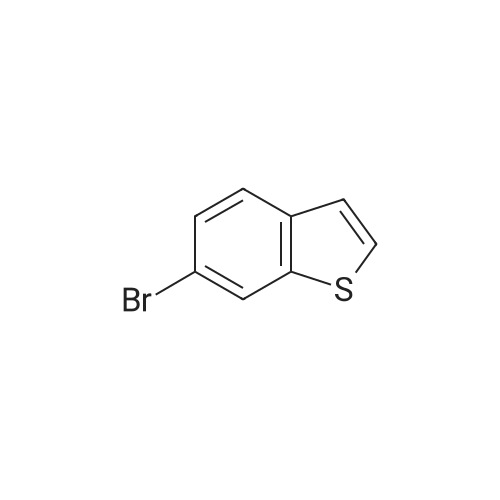
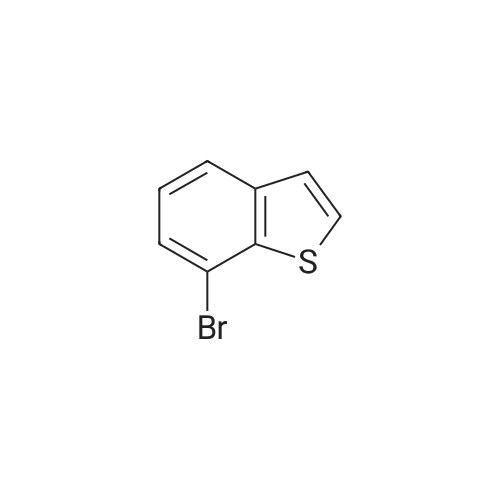
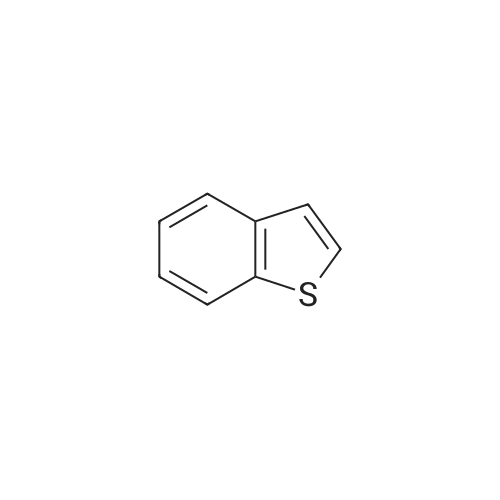
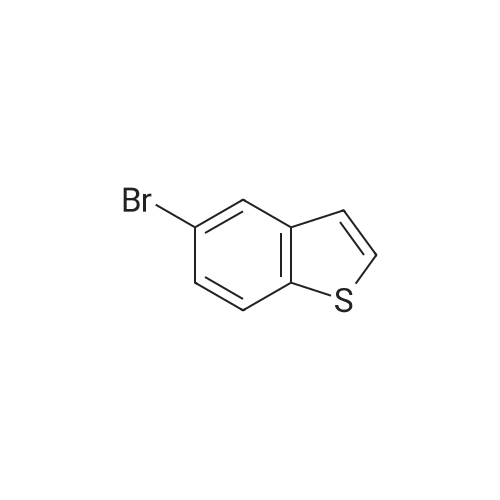
Dissolve 5-bromo-benzo [b] thiophene (J. Mater. Chem., 10: 2069-2081,2000 ; 49 g, 7.0 mmol) in DMSO (40 mL). Add bis (pinacolato) diboron (7 mmol), PdCl2 (dppf)-CH2Cl2 (0.33 mmol), and KOAc (20 mmol). Flush the flask with N2, and then heat the reaction mixture to 80C with stirring. Continue to heat the reaction mixture for 3 hours, and then cool to room temperature. Add water (66 mL) and extract the aqueous layer with EtOAc (3 x 66 mL). Combine the organic layers and dry with Na2SO4, filter, concentrate and purify by flash column chromatography (silica gel, 0-5% Et20/pentane) to give 1.56 g of the title compound (86%). |
| 64% |
With potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In dimethyl sulfoxide; at 85℃; for 1.5h; |
Step E: Bis(pinacolato)diboron (1.3 g, 5.12 mmol), dichloro[1,1'-bis(diphenylphosphino)ferrocene]palladium(II)dichloromethane (0.1 g, 0.14 mmol) and potassium acetate (1.4 g, 14.26 mmol) were charged in a 100 ml 3 neck round-bottomed flask which had been dried with a heat gun under vacuum and cooled under nitrogen prior to use. The mixture was degassed with nitrogen three times. A solution of 5-bromothiophene (1.0 g, 4.69 mmol) in dimethyl sulfoxide (20 ml) was added, the mixture was degassed again three times and was stirred at 85 C. for 1.5 hours. After cooling to room temperature, the mixture was filtered through diatomaceous earth and rinsed with water and ethyl acetate. Additional water was added to the filtrate which was extracted with ethyl acetate three times. The combined organic extracts were washed with brine once and dried over sodium sulfate to give the desired material (0.8 g, 64%, 100% AUC GC) after chromatography (9:1 to 5:1 heptane/ethyl acetate): 1H NMR (300 MHz, CDCl3) delta 8.24 (s, 1H), 7.81 (d, J=8.1 Hz, 1H), 7.68 (dd, J=8.1, 0.6 Hz, 1H), 7.34 (d, J=5.4 Hz, 1H), 7.28 (dd, J=5.4, 0.6 Hz, 1H), 1.30 (s, 12H). |
|
With potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In dioxane 1-4; at 100℃; for 20h; |
EXAMPLE 10A 2-Benzo[b]thiophen-5-yl-4,4,5,5-tetramethyl-[3.2.1]dioxaborolane A mixture of 5-bromo-benzo[b]thiophene (Maybridge, 4.26 g, 0.0200 mol), bis(pinacolato)diboron (Aldrich, 6.09 g, 0.0240 mol) and potassium acetate (Aldrich, 2.94 g, 0.0300 mol) in 1,4-dioxane (Aldrich, 50 mL) was degassed and purged with N2 three times. [1,1'-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) PdCl2(dppf).CH2Cl2 (300 mg, 0.4 mmol, Aldrich) was and the solution was heated to 100 C. for 20 hours. The mixture was then cooled to room temperature, diluted with 300 mL of EtOAc and washed with brine (2*20 mL). The organic solution was concentrated under reduced pressure and the residue was chromatographed to provide the title product. 1H NMR (300 MHz, CDCl3) delta 1.36-1.41 (S, 12H), 7.35 (d, J=5.50 Hz, 1H), 7.42 (d, J=5.70 Hz, 1H), 7.75 (d, J=8.14 Hz, 1H), 7.89 (d, J=8.14 Hz, 1H), 8.31 (s, 1H) ppm. MS (DCI/NH3) m/z 278 (M+H)+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping