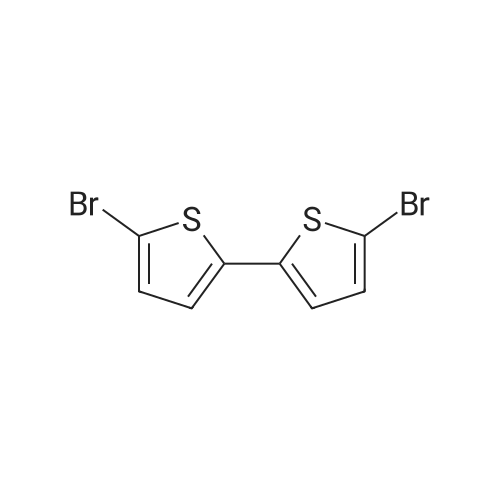
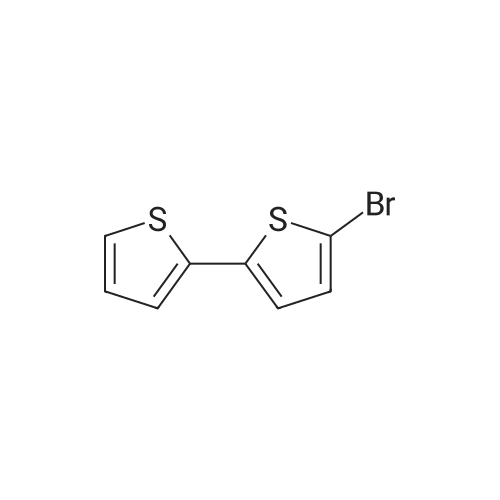
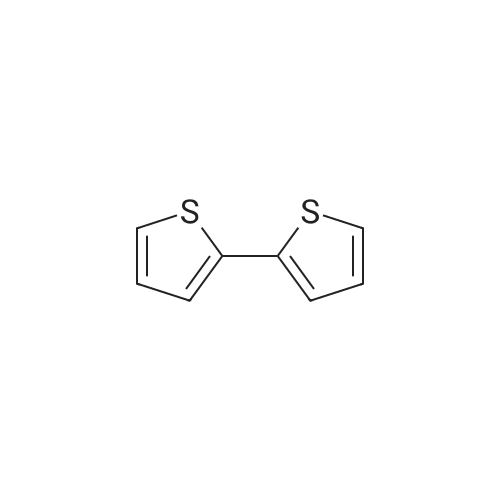
| 93% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; for 2h;Reflux; |
2,2′-bithiophene (11.7 g, 70.1 mmol) is dissolved in 500 mL of N,N-dimethylmethanamide, and N-bromo succinimide (31 g, 175.2 mmol) is added in a dropwise fashion to bromide it. Then, the resultant is refluxed for 2 hours, water (1 L) is added thereto, and the produced precipitate is filtered and recovered. The recovered powder is dissolved in chloroform (700 mL), washed with water, and then dried with magnesium sulfate followed by evaporating the resultant. The resultant is washed with n-hexane and dried to obtain Compound 1 (A yield of 93%). (0221) 1H-NMR (300 MHz, CDCl3): δ 6.96 (d, J=3.6 Hz, 1H), 6.85 (d, J=3.6 Hz, 1H) |
| 90% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide;Cooling with ice; |
To a solution of 2, 2'-bithiophene (12 mmol, 2 g) in anhydrousDMF (30 ml), NBS (24 mmol, 4.28 g) was added dropwisely (coolingwith ice-water during addition of NBS solution) and stirred for 3 h.The reaction mixture was then poured into 100 mL of ice water andthe beige solid was separated by vacuum filtration [1]. The crudewas purified with column chromatography (silica gel100e200 mesh), using hexane as eluent to yield yellow solid asproduct (3.6 g, yield 90%). |
| 85% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20℃; |
N-Bromosuccinimide (NBS) (11.65 g, 65.450 mmol) was added in small portions to a solution of 2,2'-bithiophene 1 (5 g, 29.76 mmol) in DMF at 0 C. After being stirred over night at room temperature, the reaction mixture was poured into water (200 mL) and extracted with CH2Cl2. The organic layer was thoroughly washed with water, aqueous sodium bicarbonate, brine and again with water, and then dried over Na2SO4. After removal of solvent, it was purified by column chromatography on silica gel using petroleum ether as eluant to afford 5,5'-dibromo-2,2'-bithiophene (2) (8.20 g, 85%) as a white crystal solid. GC/MS: 324(M+). 1H NMR (400 MHz, CDCl3): δ(ppm) 6.97-6.96 (d, J = 4.0 Hz, 2H), 6.86-6.85 (d, J = 4.0 Hz, 2H). |
| 85.69% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20℃; for 3h; |
2,2-Bithiophene (5.00 g, 30.1 mmol) was dissolved in N,N-dimethylformamide (50 mL). With stirring, N-bromosuccinimide (12.3 g, 69.2 mmol) was added at 0C and the reaction mixture was stirred at rt for 3 h. The mixture was poured into methanol (150 mL) and filtered to obtain a white solid product. Yield: 8.35 g (85.69%). 1H NMR (400 MHz, CDCl3): δ (ppm) 6.96 (d, J = 3.9 Hz, 2H), 6.85 (d, J = 3.9 Hz, 2H). |
| 57% |
With water; sodium bisulfate hydrate; sodium bromide; In acetonitrile; for 96h;Irradiation; |
In a 25mL reaction tube, add dithiophene (23.6mg, 0.2mmol), sodium bromide (61.6mg, 0.6mmol), sodium bisulfate hydrate (55.2mg, 0.4mmol), water (72mg, 4mmol) and Acetonitrile (2mL), stirred under the irradiation of three 2-watt LED lamps for 96 hours, after the reaction was completed, extracted, dried, filtered, concentrated, and separated by column chromatography to obtain a white solid 2v (37mg, 57%); |
| 51% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0℃; for 12h; |
In dimethyl formamide, dissolved was 2- (thiophen-2- yl) thiophene (30 g, 0.18 mol) . Then, with the light shielded, N-bromosuccinimide (70.7 g, 0.4 mol) was diluted with dimethyl formamide, and the dilution was slowly added dropwise to the solution at 0 C After 12 hours, the reaction was quenched, extracted with methylene chloride, and dried over magnesium sulfate. The solvent was removed, and the residue was purified via chromatography to obtain the desired compound, 2-bromo-5- (5-bromothiophen-2 -yl) thiophene (Compound 16) (29.8 g, yield: 51%) . |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping