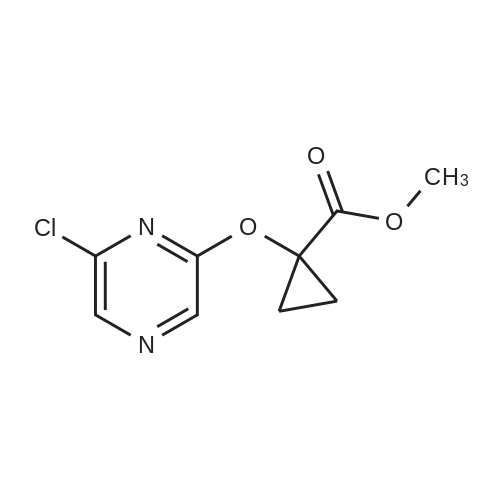
| 95% |
With ammonia; at 100℃; for 5h; |
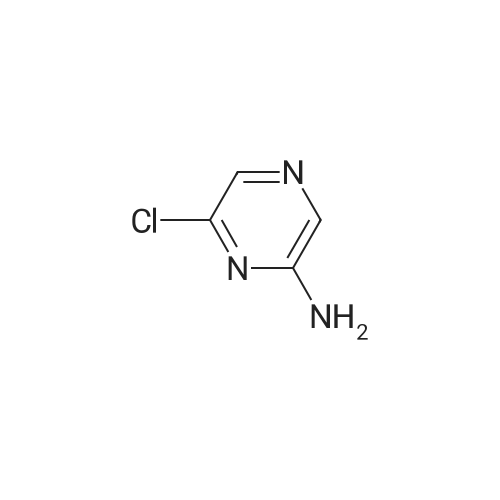
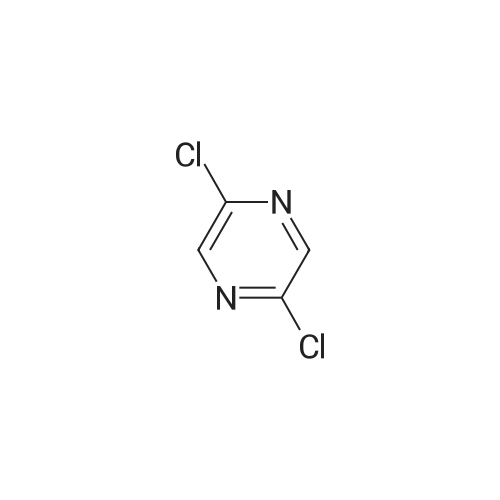
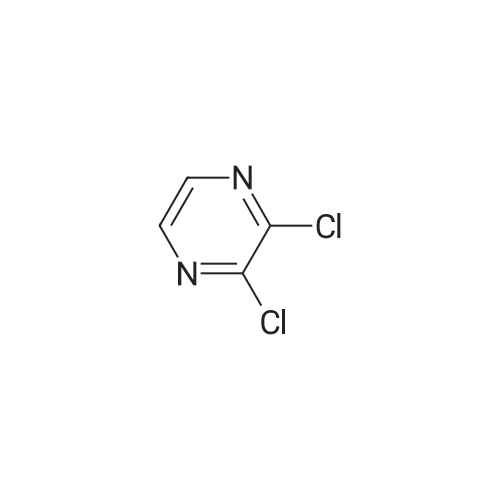
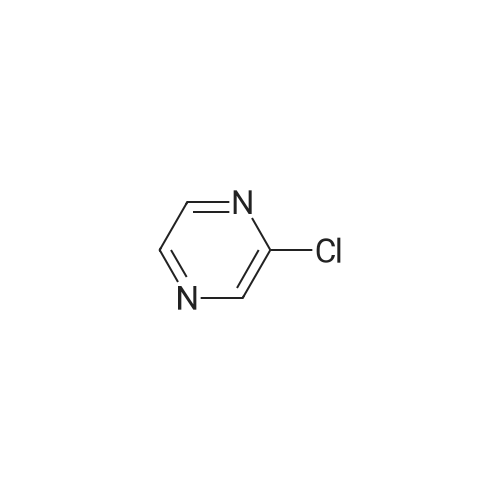
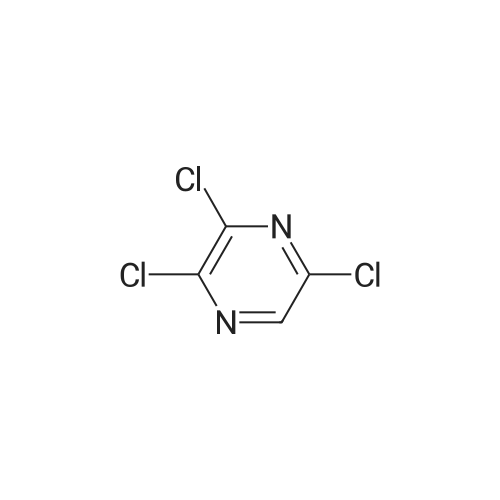
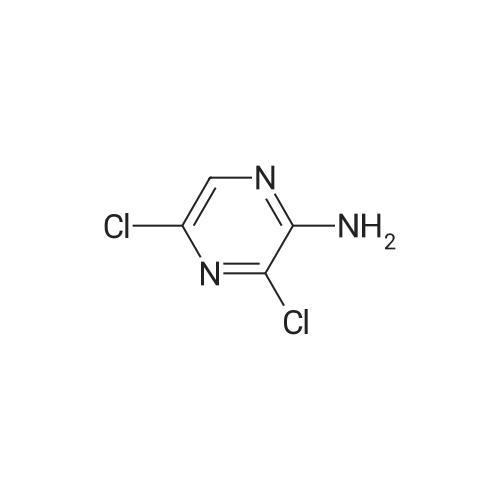
In the hydrothermal reaction kettle are respectively added with 2, 6 - two chlorine pyrrole qin (10.0 g, 67.1 mmol) and ammonia (100 ml), 100 C lower reaction 5 h. The completion of the reaction, cooling to room temperature, filtered, the filter cake is hexane (16 ml) beating shall 2 - amino -6 - chloropyrazine white solid 8.2 g, yield is 95.0%. |
| 91% |
With ammonia; In water; at 100℃; for 18h;Sealed tube; |
2,6-Dichloropyrazine (2.89 g, 19.4 mmol) was stirred in aqueous NH3 (28%, 10 ml.) and heated to 100C in a sealed tube for 18 hours. The reaction mixture was cooled and the resultant precipitate was filtered. Trituration with water and then ether gave 6- chloropyrazin-2-amine as a white solid (2.28 g, 17.6 mmol, 91 % yield). 1H NMR (d6-DMSO, 400 MHz) ? 6.9 (brs, 2H), 7.70 (d, J = 0.4, 1 H), 7.80 (d, J = 0.4, 1 H); LC-MS (ZQ, 6 minutes) Rt = 1.05 minutes; m/z (ESI+) 130 (M+H).6-Chloropyrazin-2-amine (2.50 g, 19.3 mmol) was stirred in CH2CI2 (60 ml.) at 0C.A/-Bromosuccinimide (2.92 g, 16.4 mmol) was added slowly and the reaction mixture was stirred at 0C for 60 minutes. The reaction mixture was filtered through celite and concentrated to give a brown oil. Purification by flash chromatography, eluting with 0-25% EtOAc-hexanes, gave 5-bromo-6-chloropyrazin-2-amine as a yellow solid (1 .69 g, 8.16 mmol, 42% yield). 1H NMR (de-DMSO, 400 MHz) ? 7.1 (brs, 2H), 7.65 (s, 1 H); LC-MS (ZQ, 4 minutes) Rt = 1.46 minutes; m/z (ESI-) 205 (M-H).A mixture of 5-bromo-6-chloropyrazin-2-amine (1 .00 g, 4.8 mmol), copper (I) iodide (914 mg, 4.8 mmol), 18-crown-6 (95 mg, 0.36 mmol) andtetrakis(triphenylphosphine)palladium (0) (83 mg, 0.072 mmol) was suspended in dry DMF (20 ml.) and a stream of nitrogen was passed through for 5 minutes. Potassium cyanide (312 mg, 4.8 mmol) was added and the mixture was stirred at room temperature for 30 minutes, then refluxed at 200C for 3 hours. The mixture was cooled, diluted with EtOAc and absorbed onto silica gel (10 g). DMF was removed by evaporation. The product was purified by flash chromatography, eluting with 1 :1 EtOAc-hexanes, to yield 5- amino-3-chloropyrazine-2-carbonitrile as a yellow solid (607 mg, 3.93 mmol, 82% yield). 1H NMR (d6 DMSO, 400 MHz) ? 7.87 (s, 1 H), 8.1 (brs, 2H); LC-MS (ZQ, 4 minutes) Rt = 1.20 minutes; m/z (ESI-) 153 (M-H). |
| 80% |
With ammonia; In water; at 135℃;Autoclave; |
A solution of compound 8 4500 g, 302 mol) in conc. aq. NH3 (3.0 L) was stirred at135C overnight in a 10 L sealed pressure vessel. TLC and LC/MS showed completeconversion of the starting material. The reaction mixture was cooled to roomtemperature and filtered to afford a white solid. The solid was washed with water (200mL x 3), and then dried to afford compound 9 (312 g, 80% yield) as a solid.1HNMR (400 MHz, DMSO-d6): 7,82 (s, 1 H), 712 (s, 1 H), 6.93 (s, 2H). MS Calcd.:129 MS Found: 130 ([M+Hj). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping