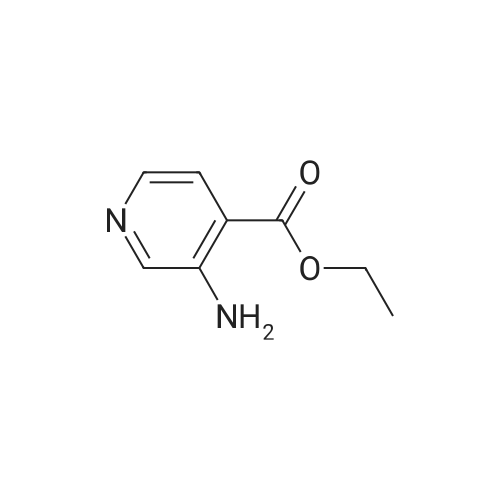
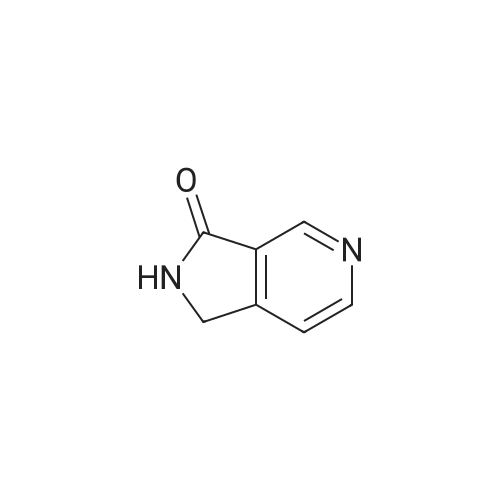
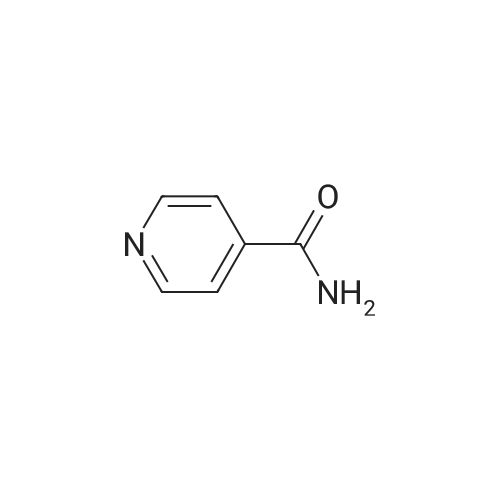
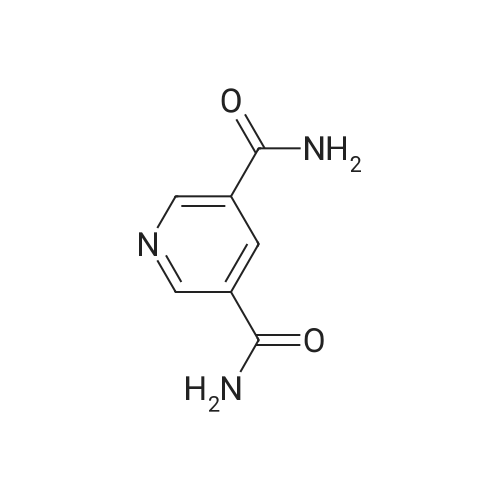
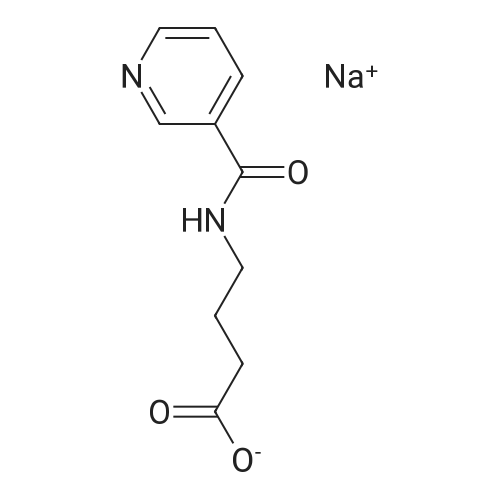
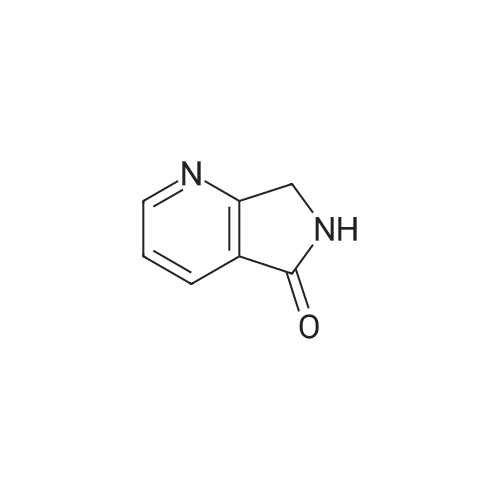
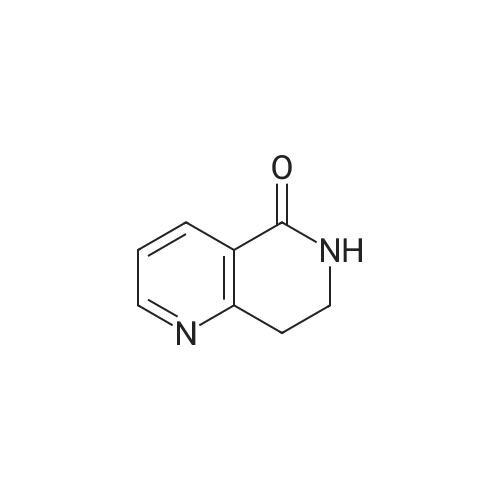
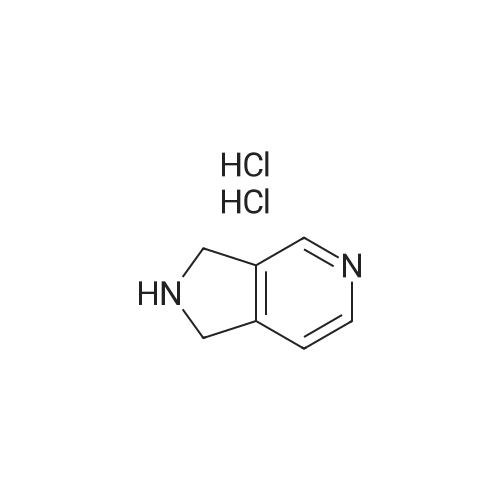
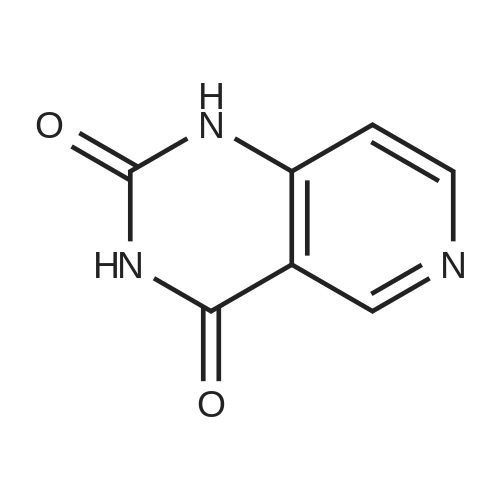
| 64% |
|
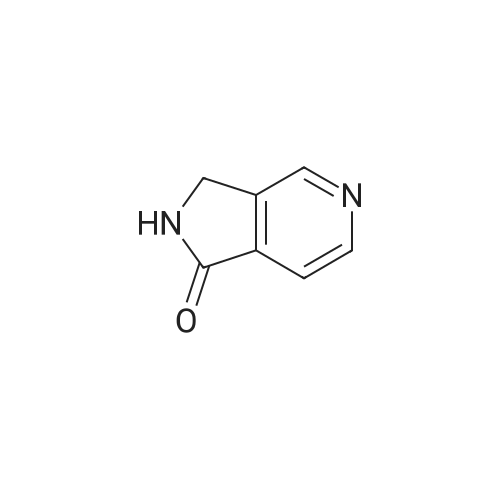
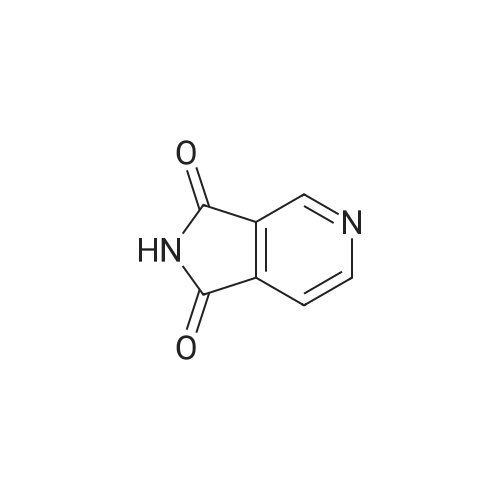
Following the procedures of Zhou et al (2001) Bioorg. Med. Chem. Lett.9(8):2061-2071, bromine (1.22 ml, 23.9 mmol) was slowly added to a cooled (50C) 2.5N solution of NaOH (60 ml, 150 mmol) and after stirring for 5 minutes pyrrolo[3,4-c]pyridine- 1,3-dione (3.5 g, 23.6 mmol) was added. The temperature was raised to 8O0C and the mixture was stirred for 1 hour before being cooled to ambient temperature. Acetic acid (5.9 ml, 98.3 mmol) was cautiously added (N.B.: gas evolution) and the solution stirred for 10 minutes whereby a precipitate formed that was collected by filtration. The solid was washed with water (20 ml) and MeOH (20 ml) then dried to afford the title compound as a yellow solid (2.1 g, 64percent). |
| 62% |
With sodium hydroxide; bromine; In water; at 0 - 80℃; for 0.75h; |
A solution of10percent aqueousNaOH (416 mL) was cooled to0 C and treated with bromine (28.2 g 176 mmol) portionwise via pipette while keeping the temperature below 5 C. To this mixture was added 3,4-pyridinedicarboximide (25.78 g, 174mmol), and the cooling bath was removed. The reaction was heated to80 C for 45 min, then allowed to cool in an ice bath. When the temperature fell to 60 C, the dropwise addition of HOAc (50 mL) was started. Cooling was continued until the temperature reached 15 C. A yellow precipitate formed. The solid was filtered, rinsed with water, then dried under vacuum to give 14.9 g of product (108 mmol,62percent). 1NMR IS-MS, m/e 139(m+1) |
| 57% |
With bromine; In sodium hydroxide; |
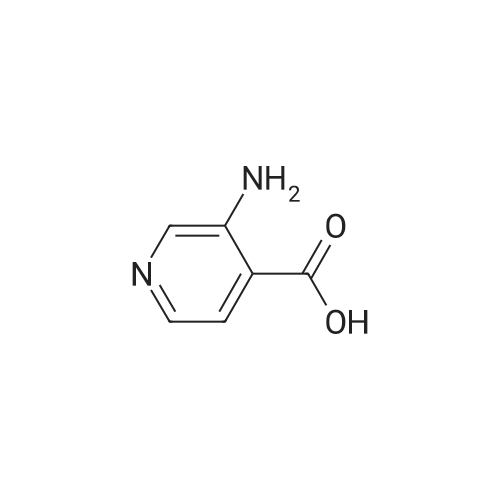
Preparation 18 3-Aminopyridine-4-carboxylic acid To an ice cold mixture of 3,4-pyridinedicarboximide (5.2 g, 35.11 mmol) in 10percent sodium hydroxide (85 mL) was added bromine (1.84 mL, 35.8 mmol), dropwise. The resulting solution was heated to 80° C. for 1 hour, cooled on ice, and the acidity was carefully adjusted to pH 5.5 with acetic acid. The precipitate was collected, washed well with water and air dried to afford 3-aminopyridine-4-carboxylic acid (2.74 g, 57percent). NMR (DMSO d6) delta8.20 (s, 1H), 7.72 (d, J=5 Hz, 1H), 7.45 (d, J=5 Hz, 1H). The material was used without purification. |
| 57% |
|
Step A: 3-Aminoisonicotinic acid: 2H-Pyrrolo[3,4-c]pyridine-1,3-dione (204.16 g, 1378.4 mmol) was dissolved in 10percent NaOH (3.3 L) and the solution was cooled to an internal temperature of 7° C. (ice/salt bath). Bromine (73.424 mL, 1433.5 mmol) was added dropwise while maintaining the internal temperature below 10° C. After completion of the addition, the reaction was heated to an internal temperature of 80-85° C. for 90 minutes. The reaction mixture was cooled to 20-30° C. in an ice bath then acetic acid (323.21 mL, 5651.2 mmol) was added dropwise. The reaction was stirred and cooled to 5° C. The solids were collected by vacuum filtration, washed with cold water then air-dried to provide the product (108.86 g, 57percent). |
| 57% |
With bromine; In sodium hydroxide; |
Preparation 18 3-Aminopyridine-4-carboxylic acid To an ice cold mixture of 3,4-pyridinedicarboximide (5.2 g, 35.11 mmol) in 10percent sodium hydroxide (85 mL) was added bromine (1.84 mL, 35.8 mmol), dropwise. The resulting solution was heated to 80° C. for 1 hour, cooled on ice, and the acidity was carefully adjusted to pH 5.5 with acetic acid. The precipitate was collected, washed well with water and air dried to afford 3-aminopyridine-4-carboxylic acid (2.74 g, 57percent). 1H NMR (DMSO d6) delta8.20 (s,1 H), 7.72 (d, J=5 Hz, 1 H), 7.45 (d, J=5 Hz, 1 H). The material was used without purification. |
|
|
Preparation 5 Step 1: 3,4 Pyridine-dicarboximide (10.0 g, 67.5 [MMOL)] was dissolved in 10percent aqueous [NAOH] (162 g) and the solution was cooled to an internal temperature [OF 7 OC] in an ice-salt bath. Bromine (3.6 ml ; 70 [MMOL)] was added dropwise. After the addition, the solution was heated for 45 min at a bath temperature of 80-85 [°C.] The yellow solution was then cooled to an internal temperature of 37 [°C,] and [GLACIAL ACOH] (17 ml) were added dropwise to a pH of 5.5. The resulting mixture was refrigerated overnight. The solid formed was filtered and washed with water (5 ml) and CH30H (5 [ML).] The reaction yielded 6.35 g. of product, m. p. 280-285 [°C] (decomp. ). |
|
With sodium hydroxide; bromine; In water; at 0 - 85℃; for 0.75h; |
3,4 Pyridine-dicarboximide 288 (10.0 g; 67.5 mmoles) was dissolved in 162 g. of 10percent aqueous NaOH and the solution was cooled to an internal temperature of 70C in an ice-salt bath. Bromine (3.6 ml; 70 mmoles) was added dropwise. After the addition, the solution was heated for 45 minutes at a bath temperature of 80-85 0C. The yellow solution was then cooled to an internal temperature of 37 0C, then 17 ml of glacial acetic acid were added dropwise to a pH of 5.5. The resulting mixture was saved overnight in a refrigerator. The solid formed was filtered and washed with 5 ml of water and 5 ml of methanol. The reaction yielded 6.35 g. of product 289 melting at 280-285 0C (decomp.). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping