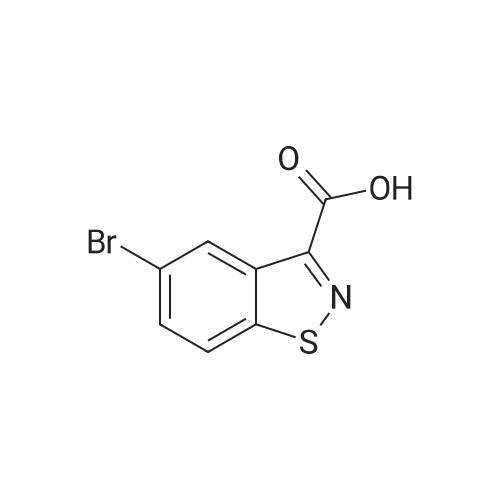
| 79% |
With diphenyl phosphoryl azide; triethylamine; for 9h;Reflux; |
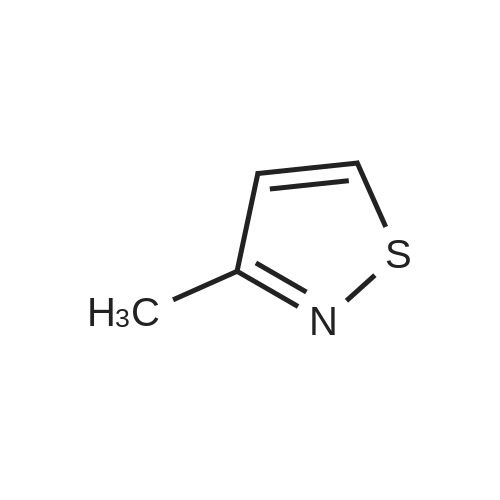
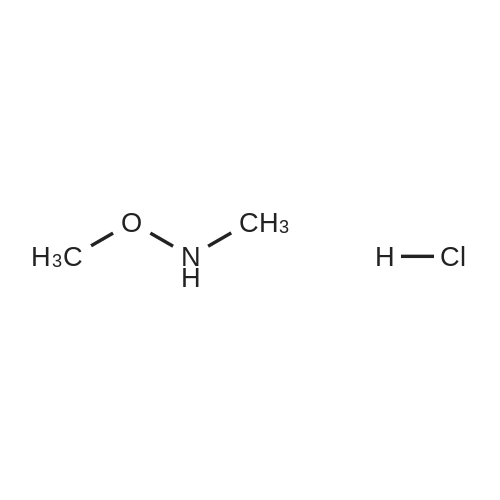
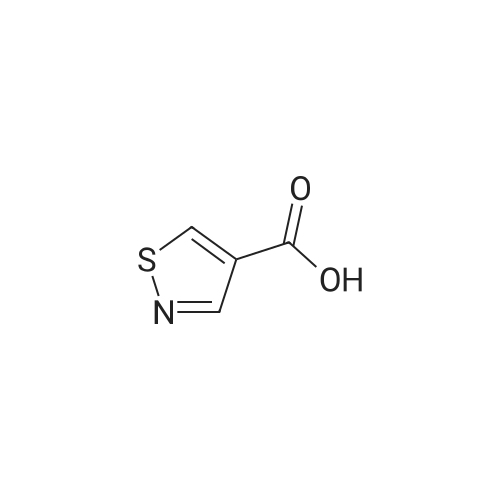
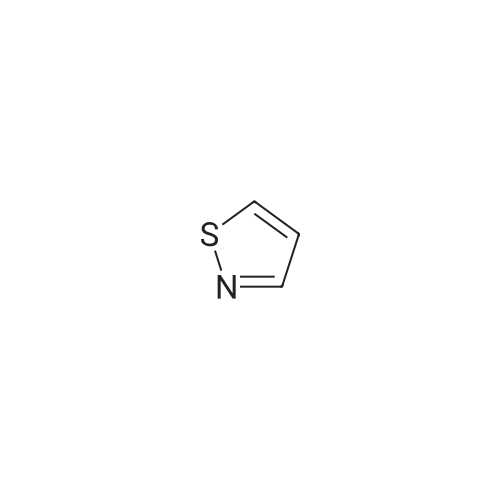
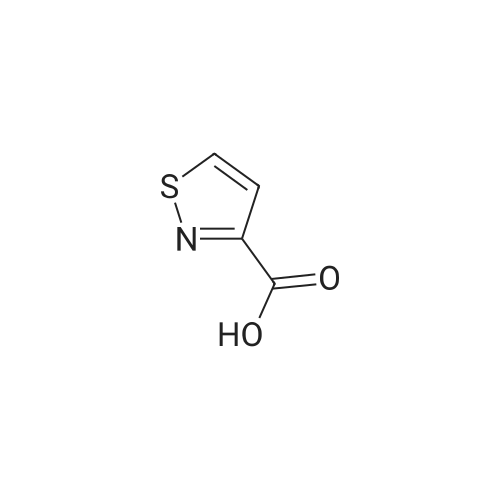
EXAMPLE 277 Synthesis of (S)-4-((1-benzylpyrrolidin-3-yl)(methyl)amino)-2-fluoro-//-(isothiazol-3-yl)- 5-methylbenzenesulfonamide 2,2,2-trifluoroacetate Step 1. Preparation of te/f-butyl isothiazol-3-ylcarbamate To a slurry of <strong>[4576-90-3]isothiazole-3-carboxylic acid</strong> (5.0 g, 38.7 mmol) in terf-butanol (194 mL) was added triethylamine (4.3 g, 42.6 mmol) followed by diphenylphosphoryl azide (11.9 g, 43.3 mmol). The reaction mixture was heated to reflux for 9 hours. Concentration under reduced pressure provided a residue which was dissolved in ethyl acetate (300 mL). The organic layer was washed with water (100 mL), 1 N sodium hydroxide solution (50 mL), water (100 mL), and brine (50 mL). The organic layer was dried over anhydrous magnesium sulfate, filtered, and the filtrate concentrated in vacuo. Purification of the residue by column chromatography, eluting with a gradient of 0 to 10% of ethyl acetate in heptane, provided the title compound as a colorless solid (6.16 g, 79 % yield): H NMR (300 MHz, CDCI3) 9.03-8.98 (m, 1 H), 8.58 (d, J = 4.9 Hz, 1 H), 7.70 (d, J = 4.9 Hz, 1 H), 1.53 (d, J = 0.7 Hz, 9H). |
| 79% |
With diphenyl phosphoryl azide; triethylamine; for 9h;Reflux; |
To a slurry of <strong>[4576-90-3]isothiazole-3-carboxylic acid</strong> (5.0 g, 38.7 mmol) in tert-butanol (194 mL) was added triethylamine (4.3 g, 42.6 mmol) followed by diphenyl phosphoryl azide (11.9 g, 43.3 mmol). The reaction mixture was heated to reflux for 9 h. After cooling the ambient temperature, the reaction mixture was concentrated in vacuo and the residue dissolved in ethyl acetate (300 mL). The organic layer was washed with water (100 mL), 1 N sodium hydroxide solution (50 mL), water (100 mL), brine (50 mL), and dried over anhydrous magnesium sulfate. Filtration and concentration of the filtrate in vacuo afforded a residue. Purification of the residue by column chromatography, eluting with a gradient of 0 to 10% of ethyl acetate in heptane, provided the title compound as a colorless solid (6.16 g, 79% yield): 1H NMR (300 MHz, CDCl3) delta9.03-8.98 (m, 1H), 8.58 (d, J=4.9 Hz, 1H), 7.70 (d, J=4.9 Hz, 1H), 1.53 (d, J=0.7 Hz, 9H). |
| 79% |
With diphenyl phosphoryl azide; triethylamine; for 9h;Reflux; |
Step 1. Preparation of tert-butyl isothiazol-3-ylcarbamate To a slurry of <strong>[4576-90-3]isothiazole-3-carboxylic acid</strong> (5.0 g, 38.7 mmol) in tert-butanol (194 mL) was added triethylamine (4.3 g, 42.6 mmol) followed by diphenylphosphoryl azide (11.9 g, 43.3 mmol). The reaction mixture was heated to reflux for 9 hours. Concentration under reduced pressure provided a residue which was dissolved in ethyl acetate (300 mL). The organic layer was washed with water (100 mL), 1 N sodium hydroxide solution (50 mL), water (100 mL), and brine (50 mL). The organic layer was dried over anhydrous magnesium sulfate, filtered, and the filtrate concentrated in vacuo. Purification of the residue by column chromatography, eluting with a gradient of 0 to 10% of ethyl acetate in heptane, provided the title compound as a colorless solid (6.16 g, 79% yield): 1H NMR (300 MHz, CDCl3) delta 9.03-8.98 (m, 1H), 8.58 (d, J=4.9 Hz, 1H), 7.70 (d, J=4.9 Hz, 1H), 1.53 (d, J=0.7 Hz, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping