| 80% |
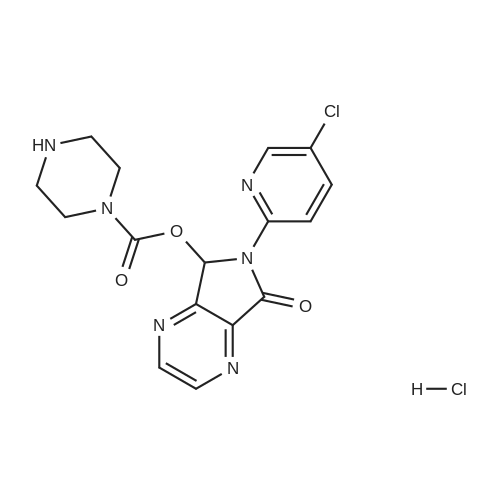
With dmap; triethylamine; In dichloromethane; at 20℃; for 3h;Reflux; |
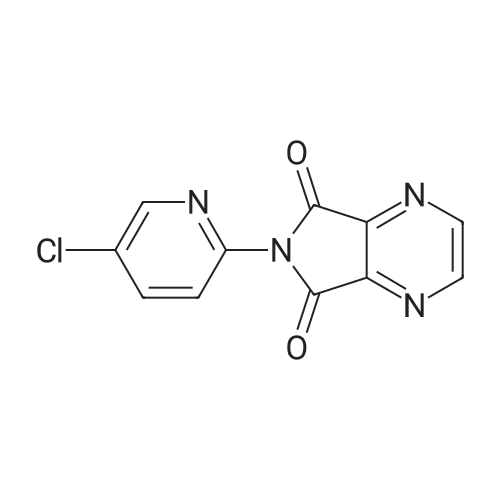
In a 500 mL three-necked flask equipped with a mechanically stirred, spherical reflux condenser,20 g of compound 4, 18.6 g of compound 5, 300 ml of dichloromethane,Anhydrous triethylamine 35mL,2. 0 g of 4-dimethylaminopyridine,After stirring at room temperature for 2 h,Reflux reaction lh,TLC indicated that the substrate reaction was complete. Naturally cooled to room temperature,Precipitation of solid,Filtration,The filtrate was washed with 1 N hydrochloric acid (50 mL x 2)The solvent was evaporated under reduced pressure,The white solid was dried and weighed 26.7 g. The solid was dissolved in 250 mL of ethyl acetate at reflux,At the same time with 2g of activated carbon decolorization,Lh after the hot filter,The filtrate cooled crystallization 4h,Filtration,To give 23. 6 g of a white solid,Yield 80%Melting point 175-177 C. |
| 78.81% |
|
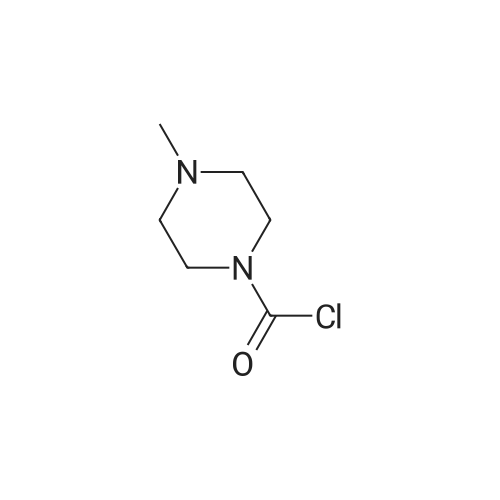
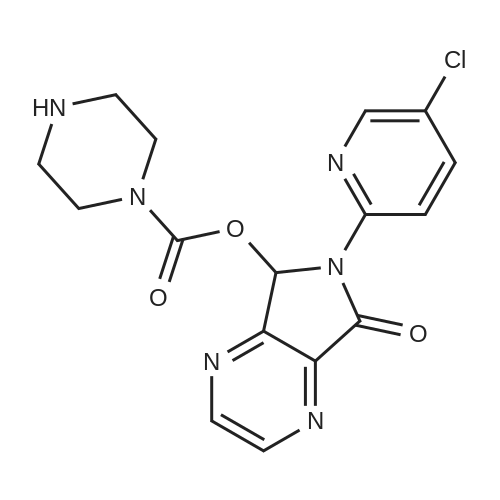
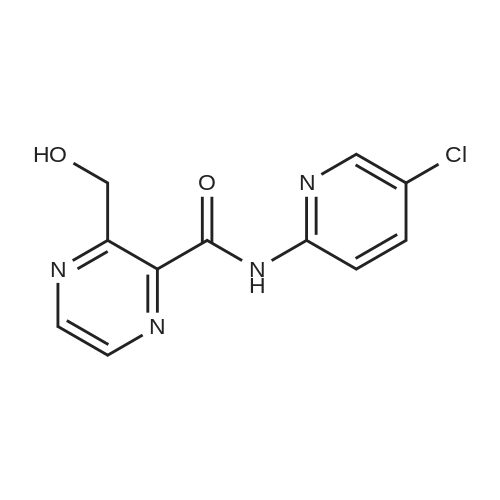
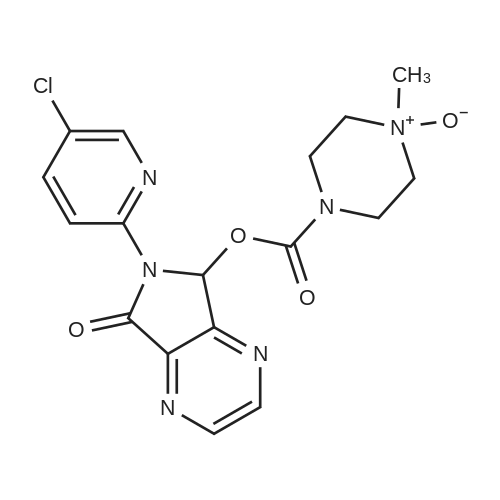
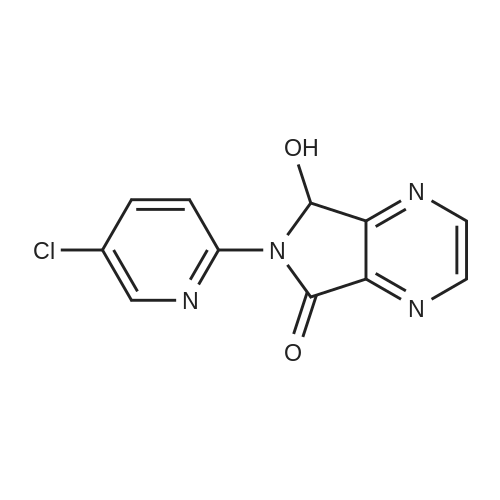
150 g (0.57 M) of 6-(5-chloropyrid-2-yl)-5-hydroxy-7-oxo-5,6-dihydropyrrolo[3,4-b]pyrazine (IV) was dissolved in 4.5 liter (30 volumes) of anhydrous N,N-dimethylformamide at 25 to 35 C. and mixture was stirred for 20 minutes. The solution was then cooled and 29.62 gm (0.74 M) of sodium hydride (50 to 60% suspension in mineral oil) was added in portions to the cooled solution with stirring. During addition, the temperature was maintained at -10 C. After complete addition, the reaction mixture was stirred at same temperature for evolution of H2 gas. A solution of 131 gm (0.8 M) of N-methyl piperazine carbonyl chloride (NMPCCl base) (III) in anhydrous N,N-dimethylformamide was slowly added. The addition was carried out at temperature of -10 C. After complete addition of NMPCCl base, the temperature was allowed to rise gradually. The mixture was stirred at temperature below 20 C. for three hours. The mixture was then quenched in 18.6 kg of ice-water and stirred for 20 minutes. Solid precipitated out was filtered off, washed with 2 liter of water, then with 1250 ml of diisopropyl ether. The product was dried at temperature of 65 C. for 18 hrs. Yield: 175 g, 78.81%. |
| 78.81% |
|
Example 2Synthesis of racemic Zopiclone (V):150 g (0.57 M) of 6-(5-chloropyrid-2-yl)-5-hydroxy-7-oxo-5,6-dihydropyrrolo [3,4- bjpyrazine (IV) was dissolved in 4.5 liter (30 volumes) of anhydrous N5N- dimethylformamide at 25 to 350C and mixture was stirred for 20 minutes. The solution was then cooled and 29.62gm (0.74 M) of sodium hydride (50 to 60 % suspension in mineral oil) was added in portions to the cooled solution with stirring. During addition, the temperature was maintained at -1O0C. After complete addition, the reaction mixture was stirred at same temperature for evolution of H2 gas. A solution of 131 gm (0.8 M) of N-methyl piperazine carbonyl chloride (NMPCCl base) (III) in anhydrous N5N- dimethylformamide was slowly added. The addition was carried out at temperature of -1O0C. After complete addition of NMPCCl base, the temperature was allowed to rise <n="21"/>gradually. The mixture was stirred at temperature below 2O0C for three hours. The mixture was then quenched in 18.6 kg of ice-water and stirred for 20 minutes. Solid precipitated out was filtered off, washed with 2 liter of water, then with 1250 ml of diisopropyl ether. The product was dried at temperature of 650C for 18 hrs. Yield: 175 g, 78.81 %.Example 13Synthesis of Eszopiclone from the recovered compound (IV):38.7 g (0.14 M) of the recovered 6-(5-chloropyrid-2-yl)-5-hydroxy-7-oxo-5,6- dihydropyrrolo [3,4-b]pyrazine (IV) was reacted with 33.79 g (0. 2 M) of N-methyl piperazine carbonyl chloride base (III) in presence of 7.64 g (0.19 M) of sodium hydride(under same conditions as that of Example 2) to yield racemic Zopiclone. RacemicZopiclone thus obtained was further converted to Eszopiclone (I) as in the foregoing examples. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping