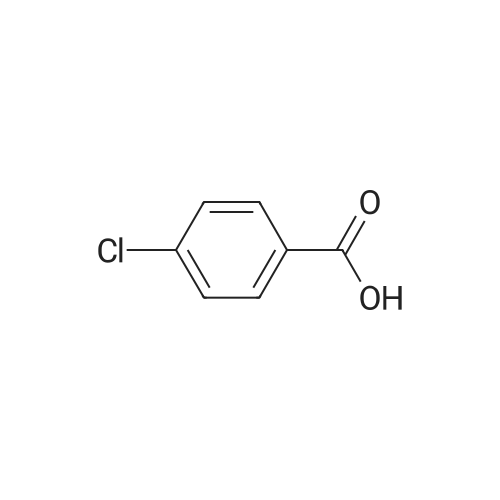
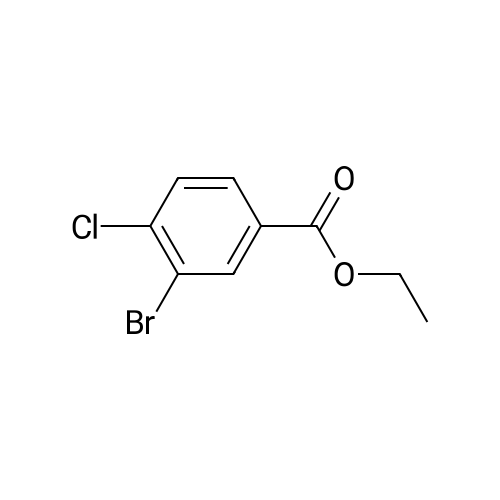
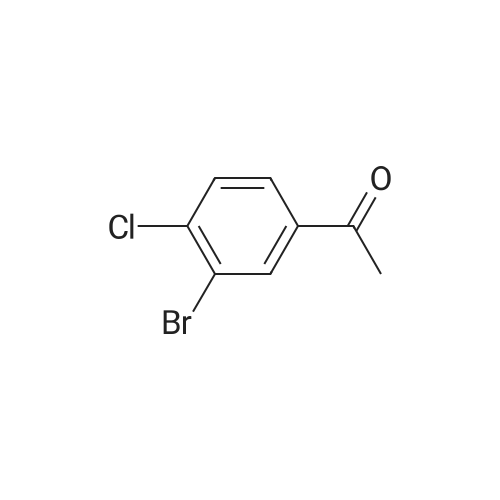
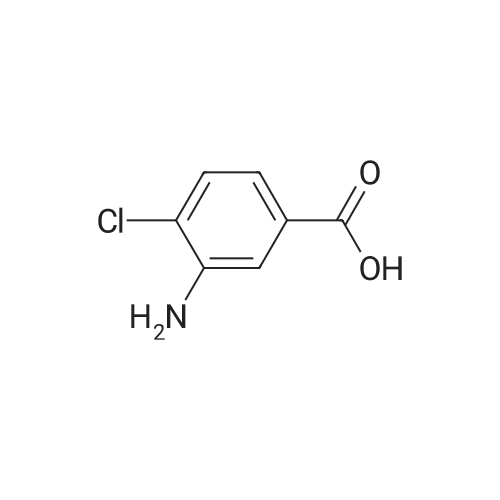
|
With oxalyl dichloride;N,N-dimethyl-formamide; In dichloromethane; at 20℃; |
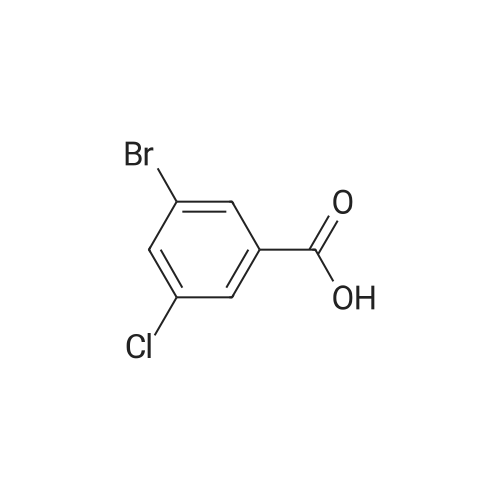
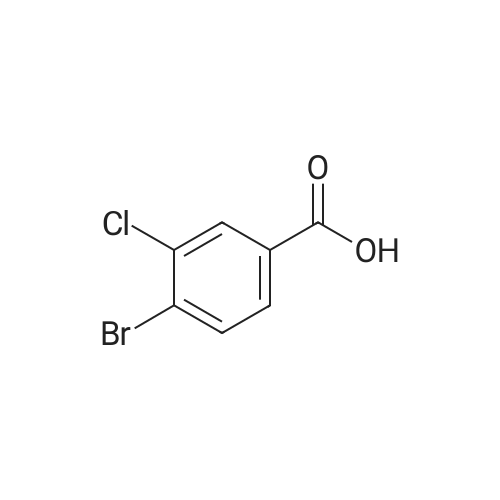
Example 77 3-Bromo-4-chlorobenzamide 3-Bromo-4-chlorobenzoic acid (2.35 g, 10 mmol) was suspended in CH2Cl2 (50 mL) and stirred under argon at room temperature. Oxalyl chloride (2.53 g, 20 mmol) was added followed by DMF (10 muL). Gas evolution began, and the mixture was stirred until gas evolution ceased. The solvents were pumped off and toluene was added and stripped off to remove excess oxalyl chloride. The residue was taken up in EtOAc and added to concentrated ammonium hydroxide (10 mL). This was stirred for 30 min. The phases were separated and the organic washed 1* with brine dried over anhydrous Na2SO4, filtered, and evaporated. The residue was crystallized from EtOAc/hexane to give the title compound as a white crystalline solid. LC-MS m/z 233.7 (M+H)+, 1.54 min (ret time); mp 146-147° C.; analytical HPLC shows 100percent purity, (ret time: 11.835 min). |
|
With oxalyl dichloride;N,N-dimethyl-formamide; In dichloromethane; at 20℃;Inert atmosphere; |
Example 94 3-Bromo-4-chlorobenzamide 3-Bromo-4-chlorobenzoic acid (2.35 g, 10 mmol) was suspended in CH2Cl2 (50 mL) and stirred under argon at room temperature. Oxalyl chloride (2.53 g, 20 mmol) was added followed by DMF (10 muL). Gas evolution began, and the mixture was stirred until gas evolution ceased. The solvents were pumped off and toluene was added and stripped off to remove excess oxalyl chloride. The residue was taken up in EtOAc and added to concentrated ammonium hydroxide (10 mL). This was stirred for thirty min. The phases were separated and the organic washed 1* with brine, dried over anhydrous Na2SO4, filtered, and concentrated. The residue was crystallized from EtOAc/hexane to give the title compound as a white crystalline solid. LC-MS m/z 233.7 (M+H)+, 1.54 min (ret time); mp 146-147° C.; analytical HPLC shows 100percent purity, (ret time 11.835 min). |
|
With oxalyl dichloride;N,N-dimethyl-formamide; In dichloromethane; at 20℃;Inert atmosphere; |
Example 77 3-Bromo-4-chlorobenzamide3-Bromo-4-chlorobenzoic acid (2.35 g, 10 mmol) was suspended in CH2Cl2 (SO mL) and stirred under argon at room temperature. Oxalyl chloride (2.53 g, 20 mmol) was added followed by DMF (10 muL). Gas evolution began, and the mixture was stirred until gas evolution ceased. The solvents were pumped off and toluene was added and stripped off to remove excess oxalyl chloride. The residue was taken up in EtOAc and added to concentrated ammonium hydroxide (10 mL). This was stirred for 30 min. The phases were separated and the organic washed IX with brine dried over anhydrous Na2SO4, filtered, and evaporated. The residue was crystallized from EtOAc/hexane to give the title compound as a white crystalline solid. LC-MS m/z 233.7 (M+H)+, 1.54 min (ret time); mp 146-147 0C; analytical HPLC shows 100percent purity, (ret time: 11.835 min). |
|
With thionyl chloride; sodium chloride; In ethyl acetate; for 5h;Reflux; Inert atmosphere; |
To a stirred suspension of <strong>[42860-10-6]3-bromo-4-chlorobenzoic acid</strong> (0.5 g) in EtOAc (10 mL) NaCl was added followed by the addition of 0.77 mL of thionyl chloride. This heterogeneous reaction mixture was then refluxed. After 5 hours, the reaction was cooled to room temperature and the volatiles were removed under reduced pressure. Excess solvent was removed and the residue was taken up in THF (10 mL) and cooled in an ice bath. Solid sodium cyanamide (0.272 g) was added to this stirred solution portionwise and the reaction was allowed to stir for twelve hours. Then THF was removed under reduced pressure and 15 mL of water was added to the residue. The solid was filtered off and the filtrate was acidified with IN aq. HC1. The precipitated white solid was isolated, filtered, and dried. Compound S3 was carried forward without additional purification. |
|
With oxalyl dichloride;N,N-dimethyl-formamide; In dichloromethane; for 1h; |
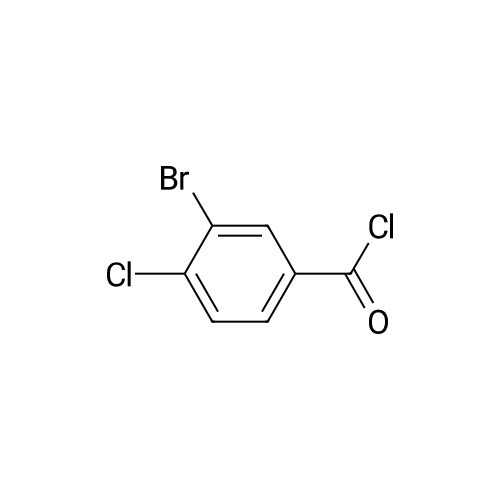
To <strong>[42860-10-6]3-bromo-4-chloro-benzoic acid</strong> (9.4 g, 40 mmol ) in dry DCM (100 mL), was added DMF (0.5 ml.) followed by addition of oxalyl chloride (22.5 ml_, 55 mmol, 2M in DCM ) slowly. The mixture was stirred for 1 hr, then concentrated to yield 3-bromo-4-chloro-benzoyl chloride as an off-white solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping