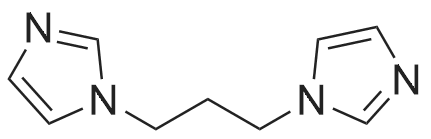
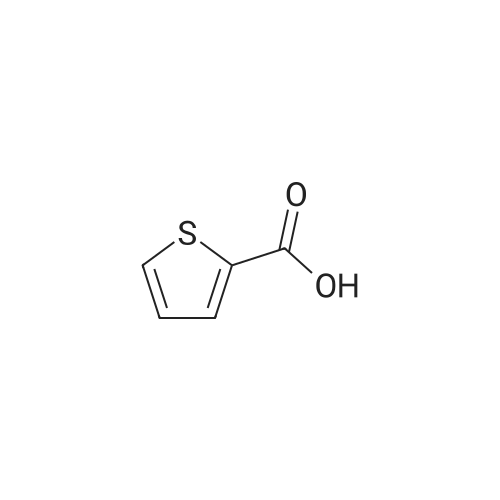
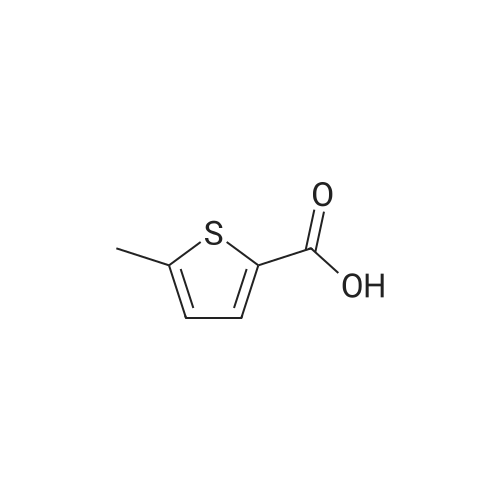
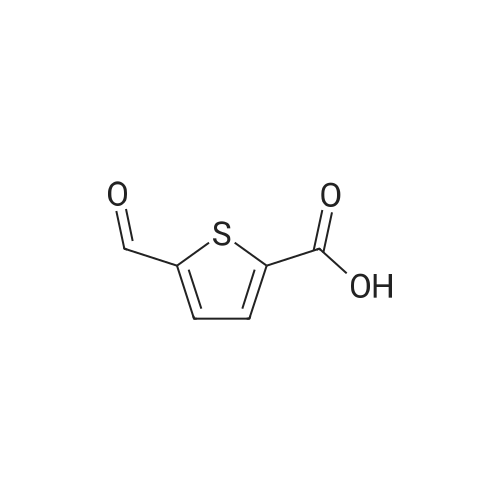
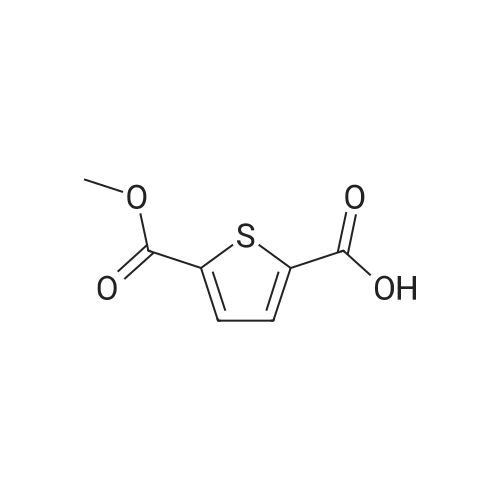
Synthesis of Bis-Thioacid Derivatives of Diarylethene and Their Photochromic Properties
Aryal, Pramod
;
Bietsch, Jonathan
;
Grandhi, Gowri Sankar
, et al.
ACS Omega,2024,9(48):47489-47499.
DOI:
10.1021/acsomega.4c05945
More
Abstract: Diarylethenes (DAEs) are an important class ofphotoswitchable compounds that typically undergo reversiblephotochemical conversions between the open and closed cyclizedforms upon treatment with UV light or visible light. In this study,we introduced thioacid functional groups to several photochromicdithienylethene (DTE) derivatives and established a method thatcan be used to prepare these photoswitchable thioacids. Fourthioacid-functionalized diarylethene derivatives were synthesizedthrough the activation of carboxylic acids with N-hydroxysuccini-mide, followed by reactions with sodium hydrosulfide with yields over 90%. These derivatives exhibited reversible photoswitchingand photochromic properties upon treatment with ultraviolet (UV) and visible lights. The thioacid groups on these compounds canact as reaction sites for attaching other desirable functionalities. The photochromic properties of these new derivatives werecharacterized by using ultraviolet?visible (UV?vis) spectroscopy. The photocyclizations of one of the derivatives and its potassiumsalt were also characterized by using nuclear magnetic resonance (NMR) spectroscopy. The anions of the thioacid formed water-soluble photochromic systems, and their applications as colorimetric sensors in agarose hydrogels were demonstrated.
Purchased from AmBeed:
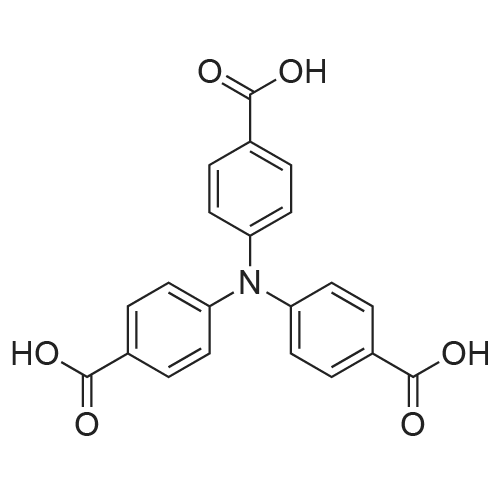
4282-31-9 ;
5798-75-4 ;
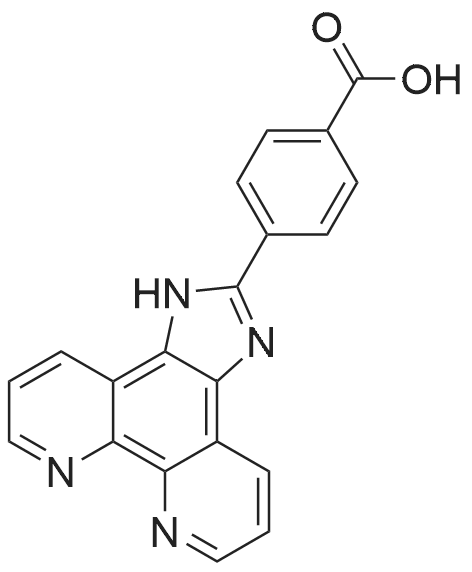
14221-01-3 ;
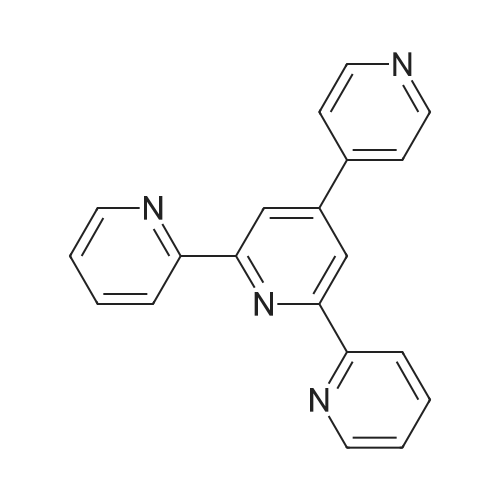
1892-57-5

Green Synthesis and Scale-Up of MOFs for Water Harvesting from Air
Zhiling Zheng
;
Ali H. Alawadhi
;
Omar M. Yaghi
Mol. Front. J.,2023,07(01n02):20-39.
DOI:
10.1142/S2529732523400011
More
Abstract: The synthetic scalability of water harvesting metal–organic frameworks (MOFs) is crucial for making these promising materials accessible and widely available for use in household devices. Herein, we present a facile, sustainable, and high-yield synthesis method to produce a series of water-harvesting MOFs, including MOF-303, CAU-23, MIL-160, MOF-313, CAU-10, and Al-fumarate. Using readily available reactants and water as the only solvent, we were able to synthesize these materials at the kilogram scale in a 200 L batch reactor with yields of 84–96% and space-time yields of 238–305 kg/day/m3 under optimized reaction conditions. We also show that our procedure preserves framework crystallinity, porosity, and water-harvesting performance of the MOFs synthesized at scale.
Keywords:
Metal-Organic Frameworks ;
Materials ;
Water Sorption ;
Green Synthesis ;
Atmospheric Water Harvesting.
Purchased from AmBeed:
4282-31-9


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping