| 151 mg (11%) |
With triethylamine; In ethanol; |
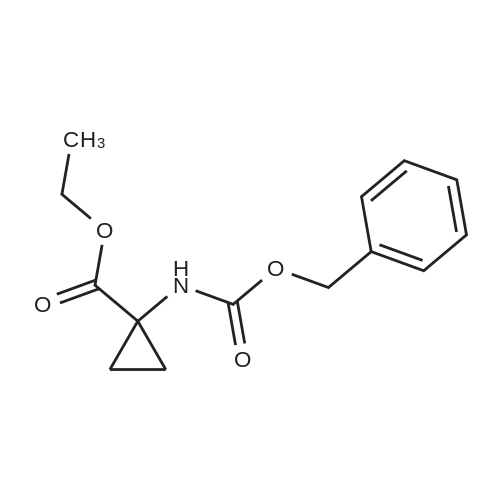
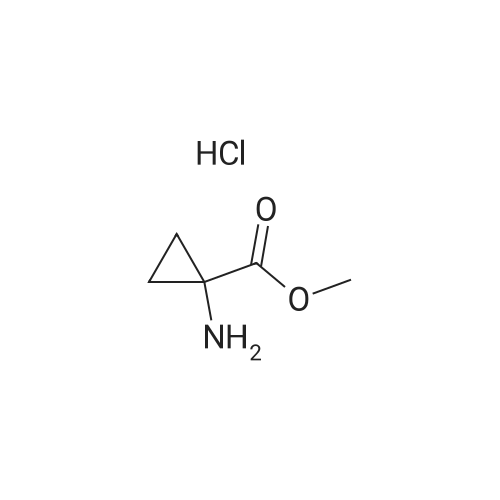
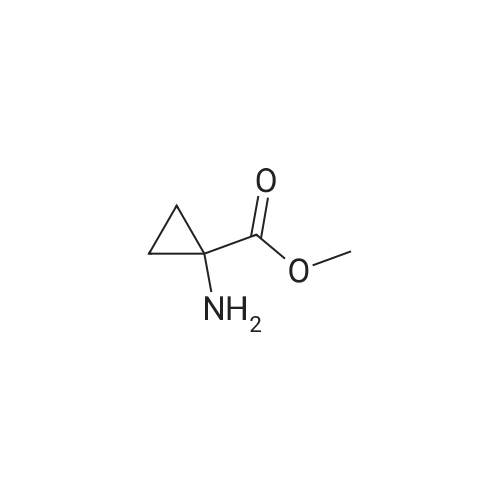
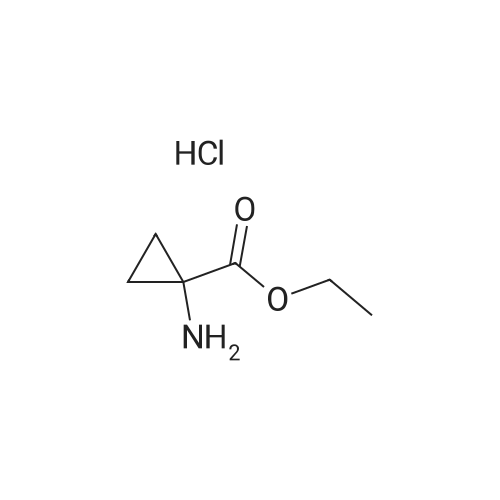
Example 7 1-(6-Chloro-1,4-dihydro-1,1-dioxo-thieno[3,2-e]-1lambda6,2,4-thiadiazin-3-ylamino)-cyclopropanecarboxylic acid ethyl ester A mixture of 3,6-dichloro-4H-thieno[3,2-e]-1,2,4-thiadiazine 1,1-dioxide (1.0 g, 3.9 mmol), <strong>[42303-42-4]1-aminocyclopropanecarboxylic acid ethyl ester hydrochloride</strong> (1.29 g, 7.8 mmol) and triethylamine (1.1 ml, 7.8 mmol) in ethanol (6 ml) was stirred for 23 h at 120C in a sealed flask.. The cooled solution was concentrated in vacuo and the residue was triturated with water followed by adjustment to PH <2 with 4M hydrochloric acid.. The resulting crude dark material was isolated by filtration and purified by chromatography (ethyl acetate) to give 151 mg (11 %) of the title compound; mp 190-194C (dec.); 1H-NMR (DMSO-d6): delta 1.15 (t, 3H), 1.22 (m, 2H), 1.50 (m, 2H), 4.09 (q, 2H), 7.06 (s, 1H), 8.14 (br s, 1H), 11.14 (br s, 1H); MS: m/e 349/351 (M+). |
| 151 mg (11%) |
With triethylamine; In ethanol; |
EXAMPLE 7 1-(6-Chloro-1,4-dihydro-1,1-dioxo-thieno[3,2-e]-1lambda6,2,4-thiadiazin-3-ylamino)-cyclopropanecarboxylic acid ethyl ester A mixture of 3,6-dichloro-4H-thieno[3,2-e]-1,2,4-thiadiazine 1,1-dioxide (1.0 g, 3.9 mmol), <strong>[42303-42-4]1-aminocyclopropanecarboxylic acid ethyl ester hydrochloride</strong> (1.29 g, 7.8 mmol) and triethylamine (1.1 ml, 7.8 mmol) in ethanol (6 ml) was stirred for 23 h at 120C in a sealed flask. The cooled solution was concentrated in vacuo and the residue was triturated with water followed by adjustment to pH <2 with 4M hydrochloric acid. The resulting crude dark material was isolated by filtration and purified by chromatography (ethyl acetate) to give 151 mg (11 %) of the title compound; mp 190-194C (dec.); 1H-NMR (DMSO-d6): delta 1.15 (t, 3H), 1.22 (m, 2H), 1.50 (m, 2H), 4.09 (q, 2H), 7.06 (s, 1H), 8.14 (br s, 1H), 11.14 (br s, 1H); MS: m/e 349/351 (M+). |
| 151 mg (11%) |
With triethylamine; In ethanol; |
Example 7 1-(6-Chloro-1,4-dihydro-1,1-dioxo-thieno[3,2-e]-1lambda6,2,4-thiadiazin-3-ylamino)-cyclopropanecarboxylic acid ethyl ester A mixture of 3,6-dichloro-4H-thieno[3,2-e]-1,2,4-thiadiazine 1,1-dioxide (1.0 g, 3.9 mmol), <strong>[42303-42-4]1-aminocyclopropanecarboxylic acid ethyl ester hydrochloride</strong> (1.29 g, 7.8 mmol) and triethylamine (1.1 ml, 7.8 mmol) in ethanol (6 ml) was stirred for 23 h at 120 C. in a sealed flask. The cooled solution was concentrated in vacuo and the residue was triturated with water followed by adjustment to pH <2 with 4M hydrochloric acid. The resulting crude dark material was isolated by filtration and purified by chromatography (ethyl acetate) to give 151 mg (11%) of the title compound; mp 190-194 C. (dec.); 1H-NMR (DMSO-d6): delta1.15 (t, 3H), 1.22 (m, 2H), 1.50 (m, 2H), 4.09 (q, 2H), 7.06 (s, 1H), 8.14 (br s, 1H), 11.14 (br s, 1H); MS: m/e 349/351 (M+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping