Alternatived Products of [ 423-62-1 ]
Product Details of [ 423-62-1 ]
| CAS No. : | 423-62-1 |
MDL No. : | MFCD00001065 |
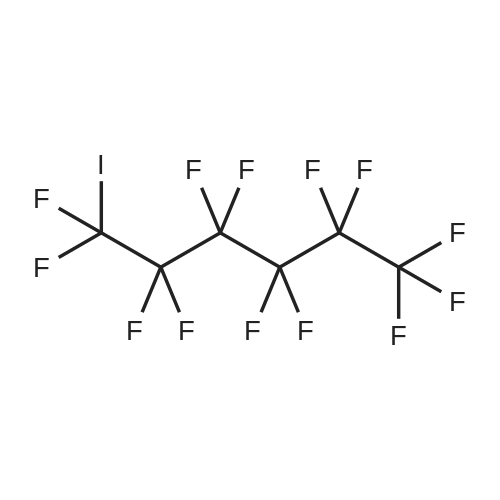
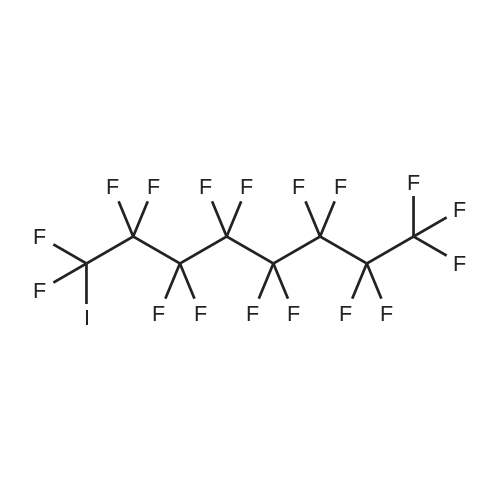
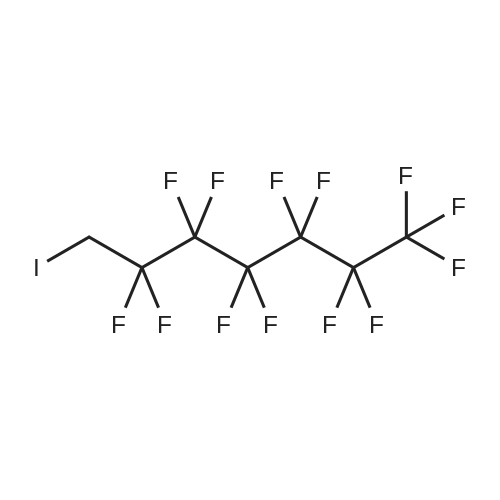
| Formula : |
C10F21I
|
Boiling Point : |
- |
| Linear Structure Formula : | I(CF2)9CF3 |
InChI Key : | UDWBMXSQHOHKOI-UHFFFAOYSA-N |
| M.W : |
645.98
|
Pubchem ID : | 67920 |
| Synonyms : |
|
Chemical Name : | Perfluorodecyl iodide |
Application In Synthesis of [ 423-62-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 423-62-1 ]
- 1
-
 [ 423-62-1 ]
[ 423-62-1 ]

-
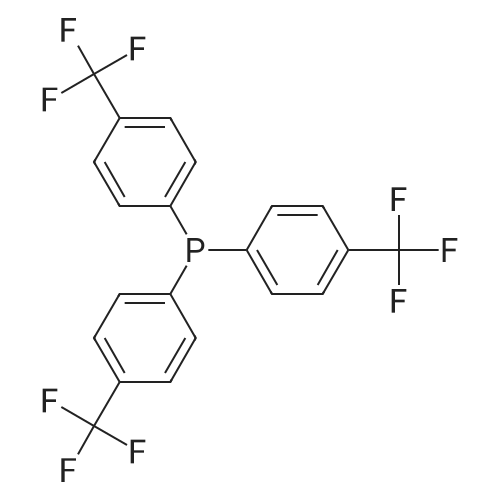 [ 13406-29-6 ]
[ 13406-29-6 ]

-
(perfluorodecyl)bis[4-(trifluoromethyl)phenyl]phosphine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 61%Spectr. |
at 15 - 25℃; for 30h;Irradiation; Sealed tube; Inert atmosphere; |
General procedure: PAr 3 (0.1 mmol), perfluoroalkyl iodide (Rf-I, 0.2 mmol), and benzotrifluoride (BTF) (1 mL) were placed in a sealed Pyrex? glass NMR tube under an argon atmosphere . The mixture was stirred for 30 seconds and then the mixture was irradiated with a xenon lamp (500 W) at room temperature (eg 15 ° C. to 25 ° C.) for 30 hours.The formation of RfPAr 2 was confirmed by 19 F NMR analysis. The crude mixture was poured into a 30 mL Schlenk tube and evaporated. MeOH (2 mL) (Wako Pure Chemical Industries, special grade, used after distillation) was added to the flask and the product was extracted with FC-72 (3 mL × 5) (3Me Japan Co., Ltd.).The product obtained was sufficiently pure without further purification. The spectrum and analytical data of 1a have already been shown in the literature (Kawaguchi et al., Angew. Chem. Int. Ed. 2013, 52, 1748-1752.). The applicable range of the substrate is shown in Table 1 |
- 2
-
 [ 423-62-1 ]
[ 423-62-1 ]

-
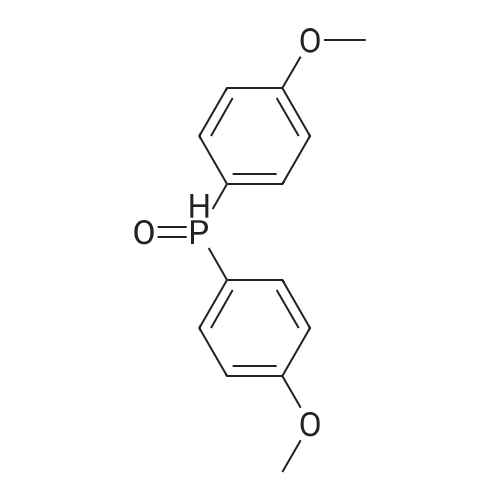 [ 15754-51-5 ]
[ 15754-51-5 ]

-
di(p-methoxylphenyl)(2,4,6-trimethylbenzoyl)phosphine oxide
[ No CAS ]

-
(perfluorodecyl)bis(4-(methoxy)phenyl)phosphine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 63% |
at 15 - 25℃; for 1.5h;Irradiation; Inert atmosphere; Sealed tube; |
General procedure: TMDPO (0.2 mmol), perfluoroalkyl iodide (nC 10 F 21 I, 0.1 mmol), Cy 2 P (O) H (0.2 mmol) and benzotrifluoride (BTF) (0.6 mL) were placed in a sealed Pyrex Registered trademark) glass tube. The mixture was stirred for 30 seconds and then the mixture was irradiated with a xenon lamp (500 W) at room temperature (eg 15 C. to 25 C.) for 1.5 hours. The formation of Compound 11 was confirmed by 19 F NMR analysis. The crude mixture was poured into a 30 mL Schlenk tube and evaporated. MeOH (2 mL) was added to the Schlenk tube and the product was extracted with FC-72 (3 mL × 5). The product obtained (fluorine-containing solvent layer) was sufficiently pure without further purification |
| 63% |
With (2,4,6-trimethylbenzoyl)diphenylphosphine oxide; at 15 - 25℃; for 1.5h;Irradiation; Sealed tube; |
TMDPO (0.2 mmol), perfluoroalkyl iodide (nC 10 F 21 I, 0.1 mmol), Cy 2 P (O) H (0.2 mmol) and benzotrifluoride (BTF) (0.6 mL) were placed in a sealed Pyrex Registered trademark) glass tube. The mixture was stirred for 30 seconds,The mixture was then irradiated with a xenon lamp (500 W) at room temperature (eg 15 C. to 25 C.) for 1.5 h. The formation of Compound 11 was confirmed by 19 F NMR analysis. The crude mixture was poured into a 30 mL Schlenk tube,Evaporated. MeOH (2 mL) was added to the Schlenk tube and the product was extracted with FC-72 (3 mL × 5). The product obtained (fluorine-containing solvent layer) was sufficiently pure without further purification. Using a derivative of TMDPO as a raw material, a perfluorophosphine compound was synthesized using the same method as in Example 1-5. The results are shown in Table 4 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping