|
With PPA; sodium bicarbonate; sulfuric acid; In ethanol; |
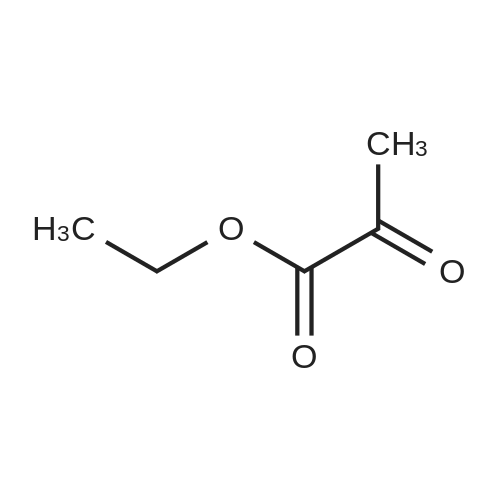
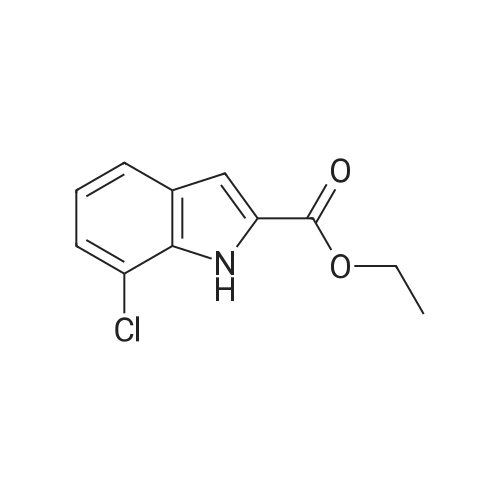
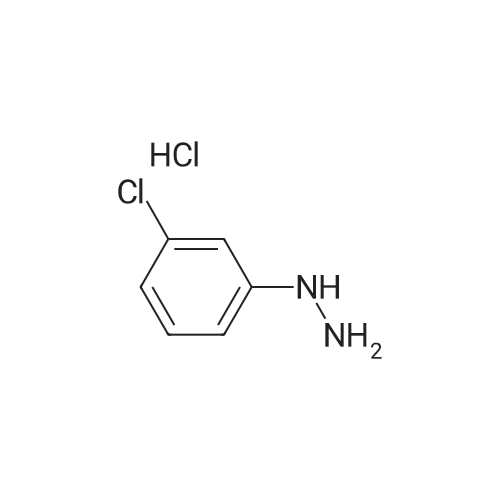
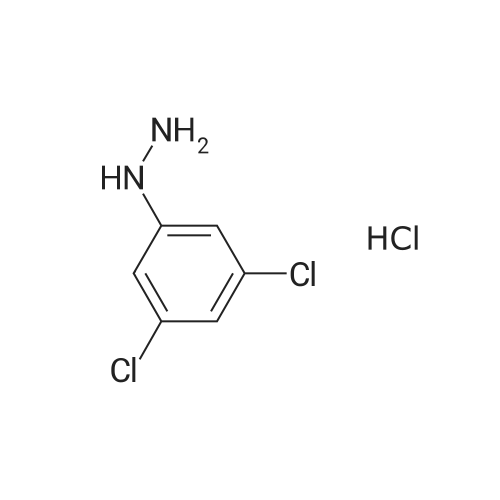
Example 39 (7-Chloro-1H-indol-2-yl)-(4-methyl-piperazin-1-yl)-methanone 2-Chlorophenylhydrazine hydrochloride (0.5 g) in ethanol (25 mL) was treated with ethylpyruvate (0.324 g) and concentrated sulfuric acid (3 drops). The mixture was stirred at ambient temperature for five min and treated with polyphosphoric acid (0.5 g). The mixture was heated at reflux temperature for 24 h whereupon additional polyphosphoric acid (0.5 g) was added and heating continued for a further 48 h. The reaction mixture was cooled to ambient temperature and concentrated under reduced pressure. The residue was partitioned between ethyl acetate and water and the pH of the aqueous layer adjusted to neutrality by addition of saturated sodium hydrogen carbonate solution. The organic fraction was separated, washed with brine, dried over magnesium sulfate, filtered, and concentrated. The residue was purified via silica gel chromatography (5-10% ethyl acetate/hexane) to give 7-Chloro-1H-indole-2-carboxylic acid ethyl ester (0.227 g). This material (0.102 g) was used without further purification. |
|
With PPA;sulfuric acid; In ethanol; at 20℃; for 72h;Heating / reflux; |
2-Chlorophenylhydrazine hydrochloride (0.5 g) in ethanol (25 mL) was treated with [ETHYLPYRUVATE] (0.324 g) and concentrated sulfuric acid (3 drops). The mixture was stirred at ambient temperature for five min and treated with polyphosphoric acid (0.5 g). The mixture was heated at reflux temperature for 24 h whereupon additional polyphosphoric acid (0.5 g) was added and heating continued for a further 48 h. The reaction mixture was cooled to ambient temperature and concentrated under reduced pressure. The residue was partitioned between ethyl acetate and water and the pH of the aqueous layer adjusted to neutrality by addition of saturated sodium hydrogen carbonate solution. The organic fraction was separated, washed with brine, dried over magnesium sulfate, filtered, and concentrated. The residue was purified via silica gel chromatography (5-10% ethyl acetate/hexane) to give [7-CHLORO-1 H-] indole-2-carboxylic acid ethyl ester (0.227 g). This material (0.102 g) was used without further purification. The ester was treated with 1 M lithium hydroxide in ethanol (5 mL) followed by water {3 mL) and stirred at ambient temperature for 18 h. The solution was acidified with 10% hydrochloric acid, diluted with water and extracted with ethyl acetate. The organic extracts were washed with brine, dried over magnesium sulfate, filtered, and concentrated to afford give 7- [CHLORO-1 H-INDOLE-2-CARBOXYLIC ACID] (0.089 g). This material (0.089 g), was treated with HATU (0.259 g), HOAT (0.093 g), N, [N-DIISOPROPYLETHYLAMINE] (0.158 mL) and N-methylpiperazine (0.05 mL) in DMF (0. [6] mL) and stirred at ambient temperature for 18 h. The reaction mixture was concentrated under reduced pressure. The residue was dissolved in ethyl acetate, washed with 1 M hydrochloric acid, saturated sodium hydrogencarbonate solution and then brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified via silica gel chromatography (2-10% 2 M ammonia in [METHANOL/DICHLOROMETHANE)] to give the title compound (0.56 g).'H NMR (400 MHz, [CDCI3)] : [8] 9.17 [(BR S, 1 H),] 7.47 (d, J = 8.1 Hz, [1H),] 7.21 (dd, J = 7.6, 0.8 Hz, 1 H), 7.01 (t, J = 7.8 Hz, 1 H), 6.73 (d, J = 2.3 Hz, 1 H), 3.88 {br m, 4H), 2.45 (t, J = 5.1 Hz, 4H), 2.29 (s, 3H). |
|
|
2-Chlorophenylhydrazine hydrochloride (0.5 g) in ethanol (25 mL) was treated with ethylpyruvate (0.324 g) and concentrated sulfuric acid (3 drops). The mixture was stirred at ambient temperature for five min and treated with polyphosphoric acid (0.5 g). The mixture was heated at reflux temperature for 24 h whereupon additional polyphosphoric acid (0.5 g) was added and heating continued for a further 48 h. The reaction mixture was cooled to ambient temperature and concentrated under reduced pressure. The residue was partitioned between ethyl acetate and water and the pH of the aqueous layer adjusted to neutrality by addition of saturated sodium hydrogen carbonate solution. The organic fraction was separated, washed with brine, dried over magnesium sulfate, filtered, and concentrated. The residue was purified via silica gel chromatography (5-10% ethyl acetate/hexane) to give 7-Chloro-1 H-indole-2-carboxylic acid ethyl ester (0.227 g). This material (0.102 g) was used without further purification. The ester was treated with 1 M lithium hydroxide in ethanol (5 mL) followed by water (3 mL) and stirred at ambient temperature for 18 h. The solution was acidified with 10% hydrochloric acid, diluted with water and extracted with ethyl acetate. The organic extracts were washed with brine, dried over magnesium sulfate, filtered, and concentrated to afford give 7-Chloro-1H-indole-2-carboxylic acid (0.089 g). This material (0.089 g), was treated with HATU (0.259 g), HOAT (0.093 g), N, N-diisopropylethylamine (0.158 mL) and N-methylpiperazine (0.05 mL) in DMF (0.6 mL) and stirred at ambient temperature for 18 h. The reaction mixture was concentrated under reduced pressure. The residue was dissolved in ethyl acetate, washed with 1 M hydrochloric acid, saturated sodium hydrogencarbonate solution and then brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified via silica gel chromatography (2-10% 2 M ammonia in methanol/ dichloromethane) to give the title compound (0.56 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping