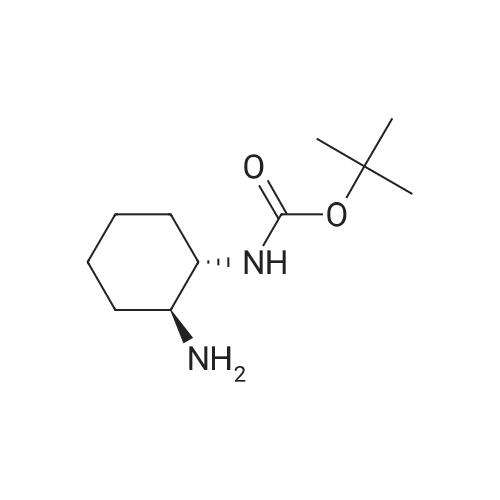
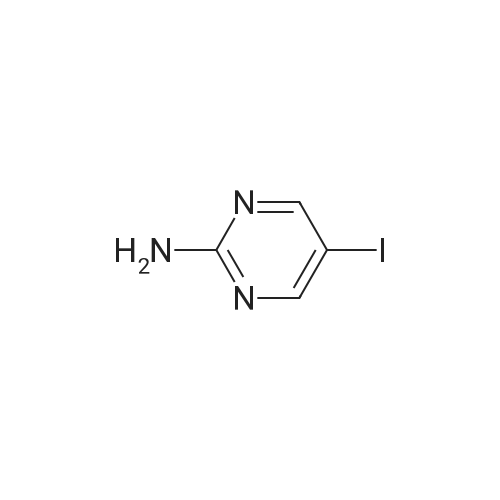
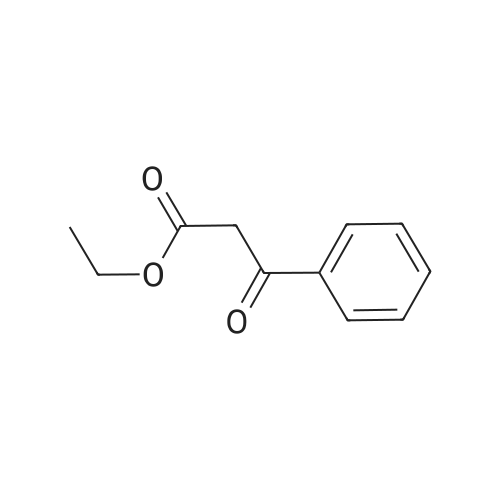
|
|
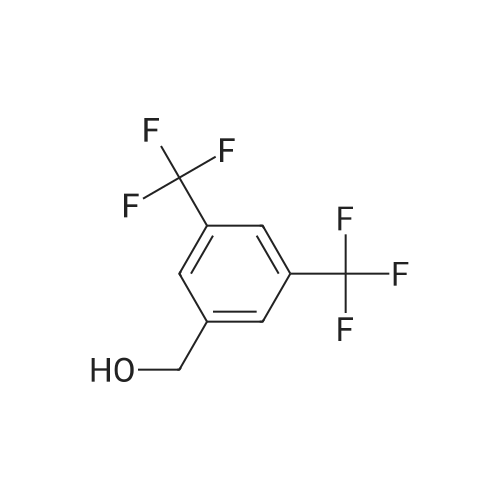
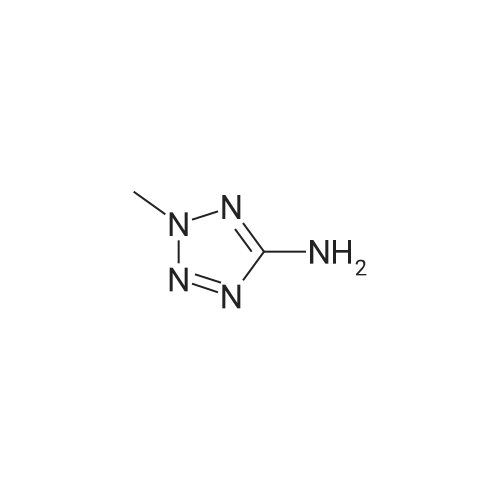
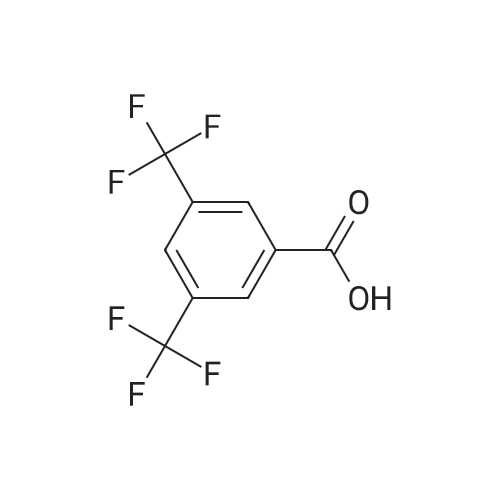
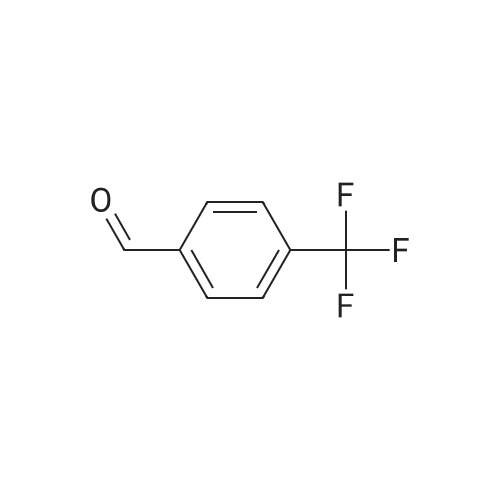
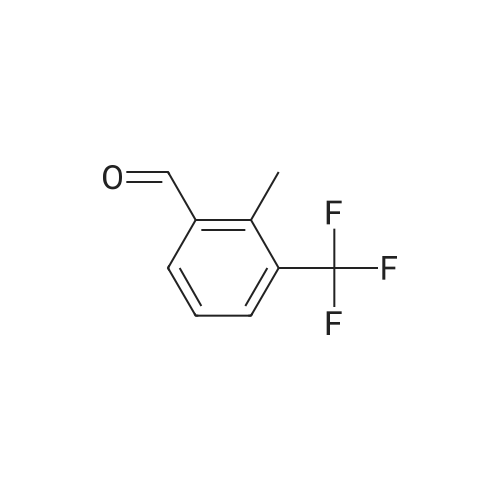
A mixture of 3,.5-bis(trifluoromethyl)benzaldehyde (4 g, 16.5 mmol), <strong>[6154-04-7]2-methyl-2H-tetrazol-5-amine</strong> (1.96 g, 19.8 mmol) and Molecular Sieves (5-10A beads) in toluene (50 ml.) was heated at reflux for 4 hours, after which time the solvent was removed. Ethanol (50 mL) and sodium borohydride (1.25 g, 33 mmol) were added. The resulting mixture was stirred at room temperature for 30 minutes, and then partitioned between saturated ammonium chloride (50 mL) and ethyl acetate (twice at 50 mL). The combine organic layers were washed with saturated NaCI (50 mL), dried (magnesium sulfate), filtered and concentrated to yield the title compound as a white solid (4.7 g). 1H NMR (300 MHz, CHLOROFORM-D) delta ppm 4.2 (s, 3 H) 4.7 (s, 1 H) 4.7 (s, 1 H) 5.0 (t, J=6.0 Hz, 1 H) 7.8 (s, 1 H) 7.9 (s, 2 H); MS (ES+) CaIc: 325.08, Found: 325.8 (M+1). |
|
|
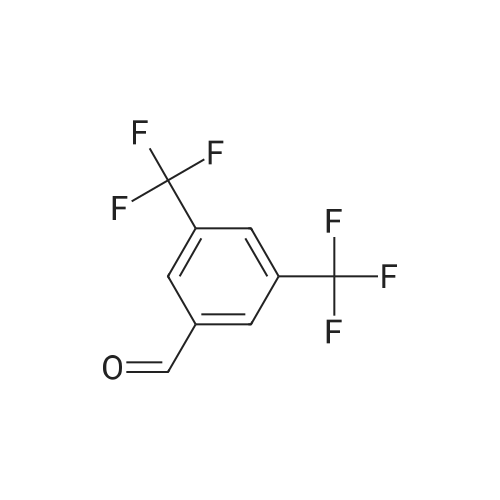
A stirred solution of 3,5~bis(trifluoromethyl)benzaldehyde (877 muL, 5.29 mmol) and 2-methyl-2H- tetrazol-5 -amine (628 mg, 6.35 mmol) in toluene (15 mL) was heated at reflux for 2.5 h. The reaction was concentrated in vacuo and the residue was redissolved in EtOH (15 mL). Sodium borohydride (400 mg, 10.58 mmol) was added and the mixture was stirred at room temperature for 14 h. The reaction was quenched with sat. NH4Cl and was partitioned between H2O (25 mL) and EtOAC (25 mL). The aqueous layer was re-extracted with EtOAc (3 x 25 mL) and the combined extracts were washed with brine (50 mL), dried (Na2SO4), filtered and concentrated in vacuo. The residue was recrystallized from IPAiH2O (3:7) and cooled at 4C for 14 h. A precipitate formed and was collected by filtration and dried in a vacuum oven to afford N-[3,5-bis(trifluoromethyl)benzyl]-2-methyl-2H-tetrazol-5-amme as a white solid. LCMS = 326.1 (M+l)+. lEta NuMR (CDCI3, 500 MHz): delta 7.86 (s, 2 H), 7.82 (s, 1 H), 4.69 (s, 2 H), 4.19(s, 3 H). |
|
|
Preparation 5 N-(3,5-Bis(trifluoromethyl)benzyl)-<strong>[6154-04-7]2-methyl-2H-tetrazol-5-amine</strong> A mixture of 3,5-bis(trifluoromethyl)benzaldehyde (4 g, 16.5 mmol), <strong>[6154-04-7]2-methyl-2H-tetrazol-5-amine</strong> (1.96 g, 19.8 mmol) and molecular sieves (5-10 A beads) in toluene (50 mL) was heated at reflux for 4 hours, after which time the solvent was removed. Ethanol (50 mL) and sodium borohydride (1.25 g, 33 mmol) were added. The resulting mixture was stirred at room temperature for 30 minutes and then partitioned between saturated NH4Cl (50 mL) and ethyl acetate (2*50 mL). The combined organic layers were washed with saturated NaCl (50 mL), dried (MgSO4), filtered and concentrated to yield the title compound as a white solid (4.7 g). 1H NMR (400 MHz, CDCl3) delta 4.2 (s, 3H) 4.7 (s, 1H) 4.7 (s, 1H) 5.0 (t, J=6.0 Hz, 1H) 7.8 (s, 1H) 7.9 (s, 2H). MS (ES+) Calc: 325.08, Found: 325.8 (M+1). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping