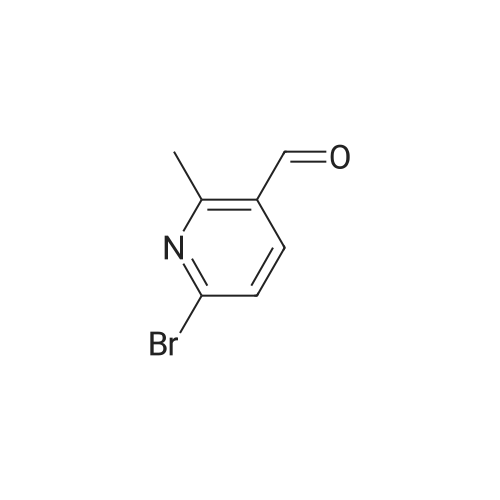
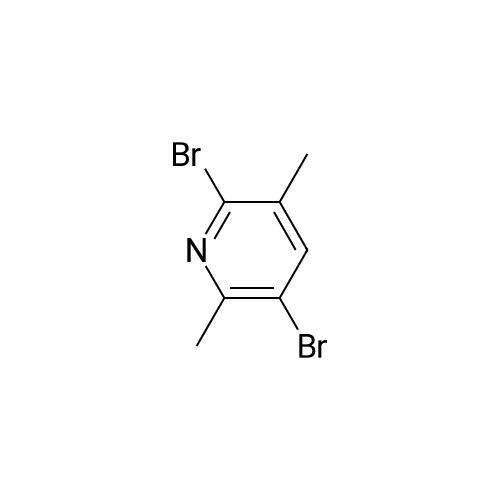
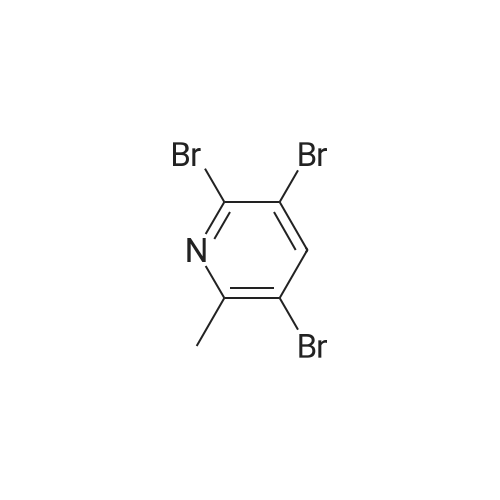
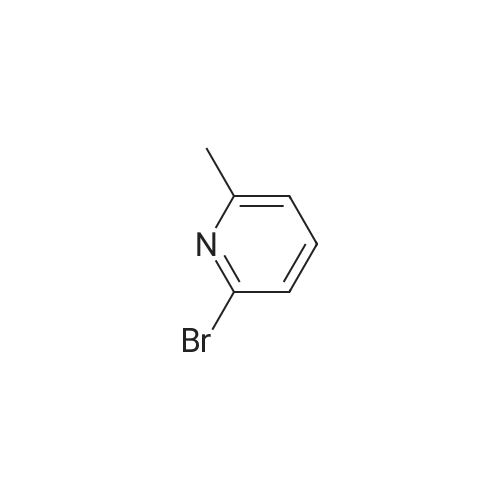
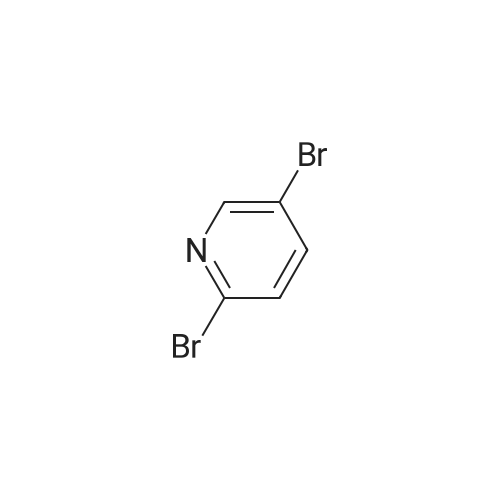
| 76% |
Stage #1: With n-butyllithium In hexanes; diethyl ether at -78℃; for 1 h;
Stage #2: at -78 - 20℃; for 2 h; |
Under N2, to a solution of 2,5-dibromo-6-methylpyridine (35.5 g, 141.1 mmol) in anhydrous Et2O (600 mL), cooled to -78 0C, was added BuLi (2.5 M in hexanes, 64.8 mL, 162 mmol) slowly, forming a yellow suspension. After addition the mixture was stirred at that temperature for 1 h, and then anhydrous DMF (18.3 g, 250 mmol) was added. After the mixture was stirred at -78 0C for 1 h, it was brought to room temperature and stirred for an additional 1 h. Aqueous HCl (0.5 N, 300 mL) was added and the organic layer was collected. The aqueous layer was extracted with EtOAc (3 x 150 mL). The combined extracts were dried (Na2SO4), filtered and the solvent was removed. The residue was purified by flash chromatography on silica gel (EtOAc/hexanes, 1 :3 in v/v) followed by recrystallization form CH2Cl2/hexanes, affording a pale yellow solid (21.5 g, 76percent). 1H NMR (CDCl3) δ 2.87 (s, 3H), 7.51 (d, IH, J= 8.1 Hz), 7.93 (d, IH, J= 8.1 Hz), 10.30 (s, IH). |
| 51% |
Stage #1: With n-butyllithium In diethyl ether; hexane at -78℃; for 1 h;
Stage #2: at 20℃; for 1 h; |
Add to the reaction flask3,6-dibromo-2-methylpyridine (1.0 g, 3.9 mmol)And anhydrous ether (10 mL) were cooled to -78°C,N-BuLi (1.6 M in hexanes, 2.4 mL, 3.9 mmol) was added dropwise,After stirring for 1 hour, anhydrous DMF (307 mg, 4.2 mmol) was added,The reaction was stirred at room temperature for 1 hour. Saturated ammonium chloride solution (10 mL) was added,Ethyl acetate (20 mL x 3). Combine organic phase,Saturated sodium chloride solution (20mL), dried over anhydrous sodium sulfate, suction filtration,Concentrated under reduced pressure. The residue was purified by column chromatography (ethyl acetate / petroleum ether = 1: 10)The resulting residue was purified to give the title compound (0.4 g, white solid) in 51percent yield. |
| 42.5% |
Stage #1: With n-butyllithium In tetrahydrofuran at -78℃; for 1 h; Inert atmosphere
Stage #2: at -78 - 25℃; for 1 h; Inert atmosphere |
To a solution of 3,6-dibromo-2-methylpyridine (60 g, 239 mmol) in THF (600 mL) and was added -BuLi (100 mL, 251 mmol) at -78 °C. The mixture was then stirred for 1 h at -78 °C under N2 atmosphere. DMF (20.4 mL, 263 mmol) was added dropwise to the mixture. The mixture was then stirred for 1 h at -78 °C under N2 atmosphere. The reaction mixture was warmed to 25 °C and 1 M aq. HC1 solution (300 mL) was added. The reaction mixture was combined with five additional crade reaction mixtures (performed at scales of 30 g, 120 mmol and 4 x 50 g, 199 mmol of 3,6-dibromo-2-methylpyridine using the same reagent and solvent stoichiometry as above) and worked up together. The reaction mixture was extracted with EtOAc (800 mL x 4). The combined organic layers were washed with H20 (300 mL x 3), dried over Na2S04, filtered, and concentrated to give 6-bromo-2-methylnicotinaldehyde (260 g, 492 mmol, 42.5percent combined yield) as a red oil. NMR (400 MHz, CHLOROFORM-d) δ 10.28 (s, 1H), 7.92 (d, .7=7.9 Hz, 1H), 7.64 (s, 1H), 2.88 - 2.83 (m, 3H). LCMS [M+H]+ = 200.1, 202.1. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping