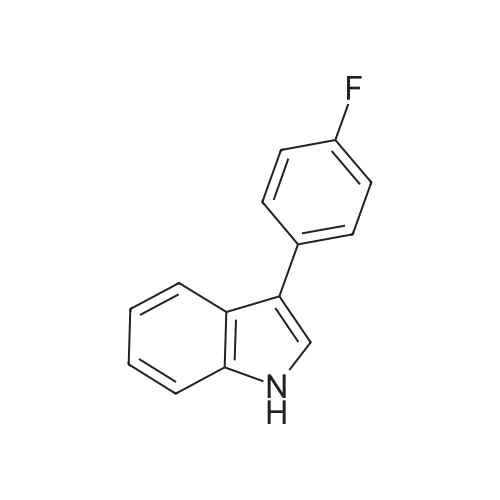
| 89% |
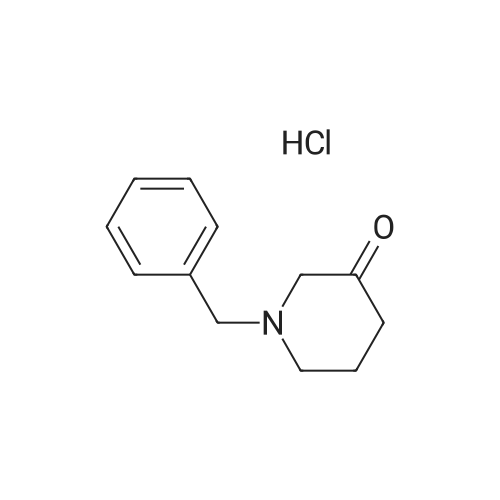
With zinc(II) chloride In acetonitrile at 85℃; for 24.1667 h; |
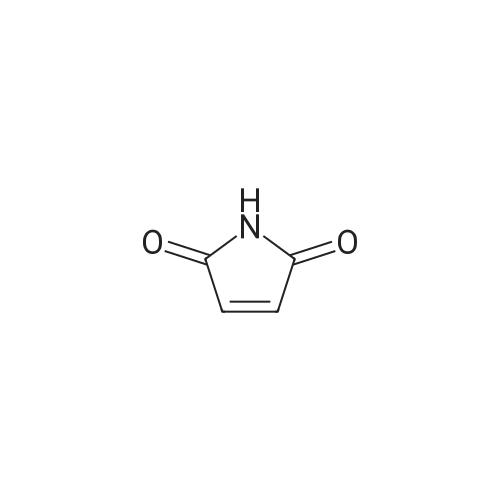
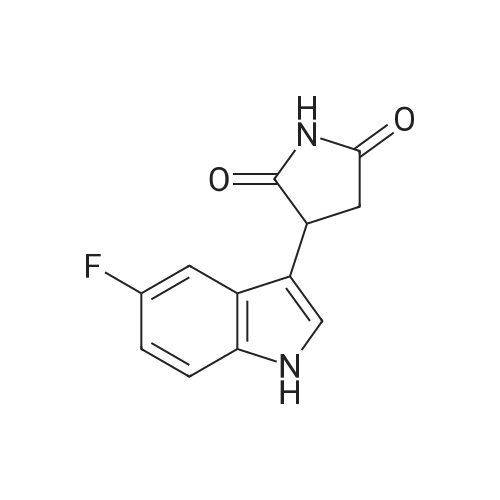
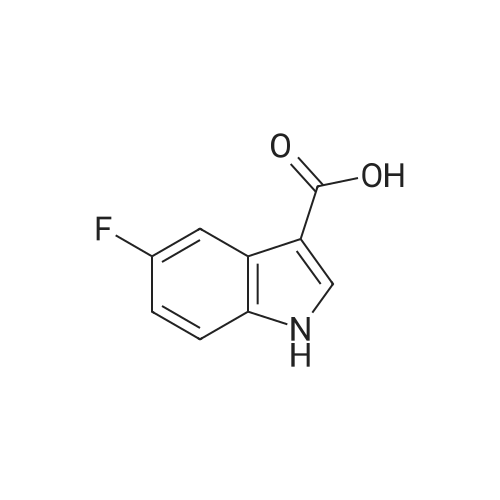
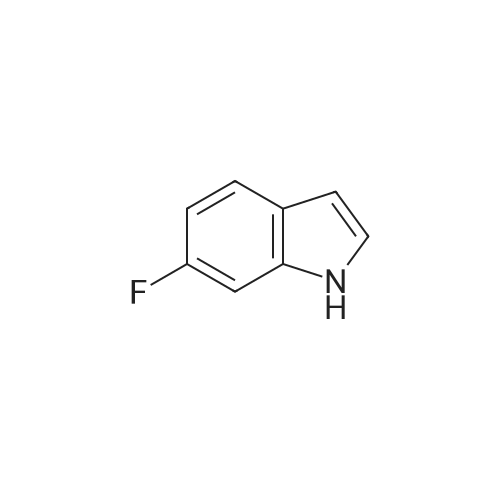
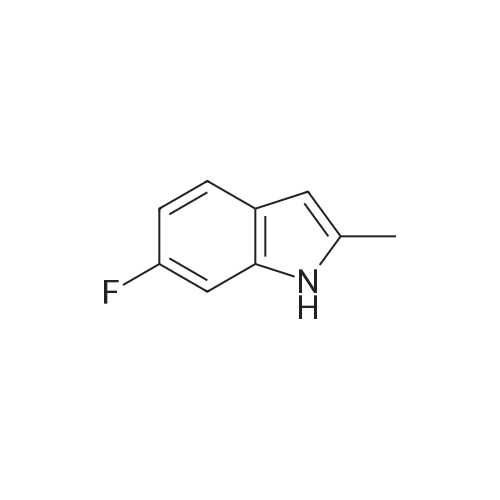
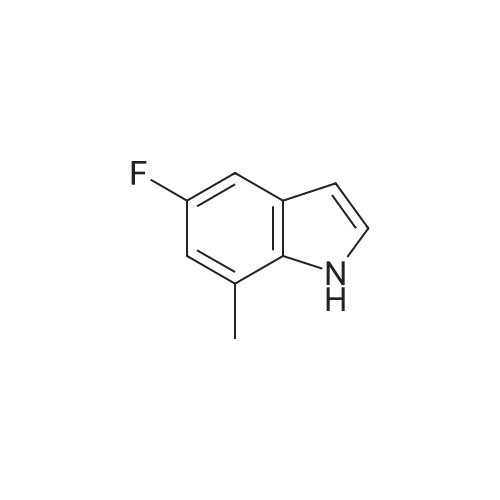
Alternatively, a mixture of 5-fluoroindole (5.00 g, 5.00 g, 35.5 mmol, 96 mass percent, 1.00) and maleimide (1.5 equiv., 5.17 g, 53.3 mmol, 1.50) was charged in a 50 mL vessel, and then acetonitrile (3 L/kg, 15.0 mL, 1 1.7 g, 286 mmol, 100 mass percent) and zinc chloride (1.05 equiv., 5.08 g, 37.3 mmol, 100 mass percent) were added. The reaction was heated to 85°C over 10 min and then maintained at 85°C for 24 hrs. While still at 85 °C, water (6 L/kg, 30.0 mL, 30.0 g, 1670 mmol, 100 mass percent) was added slowly, while maintaining the temperature above 80°C. Yellow solids precipitated. The reaction mixture was cooled to 50°C over 1 hour followed by stirring at 50°C for 2 hours, then cooled 10°C over 1 hour. The reaction was stirred at 10°C for 1 hour. The solids were filtered off, then the filter cake was washed 2 times with 5 ml 1 :1 ACN/water to afford isolated compound (6.85 g, 6.85 g, 29.5 mmol, 83.1 percent Yield). For purification, the resulting isolated compound was charged (6.85 g, 6.85 g, 29.5 mmol, 100 mass percent) into a vessel, followed by addition of tetrahydrofuran (6 L/kg, 41.1 mL, 36.4 g, 505 mmol, 100 mass percent). This mixture was heated to 66°C to form a homogeneous solution. Heptane (4 L/kg, 27.4 mL, 18.7 g, 187 mmol, 100 mass percent, was added slowly at 66°C ; solids began to precipitate after 5 volumes. The mixture was cooled to 25°C over 3 hours, then filtered and washed with heptane, followed by drying in the high vacuum oven overnight. Isolated compound (4.93 g, 4.93 g, 21.2 mmol, 100 mass percent, 72.0percent Yield). This isolated compound is charged 2 (1.00 g, 4.3 mmol, 100 mass percent,) into a 50ml vessel and tetrahydrofuran (6 L/kg, 6 mL, 100 mass percent) and heptane (6 L/kg, 6 mL, 100 mass percent) were added. The slurry was stirred at 25°C for 48 hrs. The solids were filtered off and dried in the high vacuum oven overnight. The Isolated compound : (0.89 g, 3.83 mmol, 100 mass percent, 89.00percent Yield). |
| 83.1% |
With zinc(II) chloride In acetonitrile at 85℃; for 24 h; |
Route B: (0356) Alternatively, a mixture of 5-Fluoroindole (5.00 g, 5.00 g, 35.5 mmol, 96 mass percent, 1.00) and Maleimide (1.5 equiv., 5.17 g, 53.3 mmol, 1.50) was charged in a 50 mL vessel, and then Acetonitrile (3 L/kg, 15.0 mL, 11.7 g, 286 mmol, 100 mass percent) and Zinc Chloride (1.05 equiv., 5.08 g, 37.3 mmol, 100 mass percent) were added. The reaction was heated to 85° C. over 10 min and then maintained at 85° C. for 24 hrs. While still at 85° C., Water (6 L/kg, 30.0 mL, 30.0 g, 1670 mmol, 100 mass percent) was added slowly, while maintaining the temperature above 80° C. Yellow solids precipitated. The reaction mixture was cooled to 50° C. over 1 hour followed by stirring at 50° C. for 2 hours, then cooled 10° C. over 1 hour. The reaction was stirred at 10° C. for 1 hour. The solids were filtered off, then the filter cake was washed 2 times with 5 ml 1:1 ACN/water to afford isolated compound (6.85 g, 6.85 g, 29.5 mmol, 83.1percent Yield). (0357) For purification, the resulting isolated compound was charged (6.85 g, 6.85 g, 29.5 mmol, 100 mass percent) into a vessel, followed by addition of Tetrahydrofuran (6 L/kg, 41.1 mL, 36.4 g, 505 mmol, 100 mass percent). This mixture was heated to 66° C. to form a homogeneous solution. Heptane (4 L/kg, 27.4 mL, 18.7 g, 187 mmol, 100 mass percent, was added slowly at 66° C.; solids began to precipitate after 5 volumes. The mixture was cooled to 25° C. over 3 hours, then filtered and washed with heptane, followed by drying in the high vacuum oven overnight. Isolated compound (4.93 g, 4.93 g, 21.2 mmol, 100 mass percent, 72.0percent Yield). (0358) This isolated compound is charged 2 (1.00 g, 4.3 mmol, 100 mass percent) into a 50 ml vessel And Tetrahydrofuran (6 L/kg, 6 mL, 100 mass percent) and Heptane (6 L/kg, 6 mL, 100 mass percent) were added. The slurry was stirred at 25° C. for 48 hrs. The solids were filtered off and dried in the high vacuum oven overnight. The Isolated compound: (0.89 g, 0.89 g, 3.83 mmol, 100 mass percent, 89.00percent Yield). |
| 83.1% |
Stage #1: With zinc(II) chloride In acetonitrile at 85℃; for 24 h; Microwave irradiation
Stage #2: at 10 - 80℃; for 4 h; |
Alternatively, a mixture of 5-Fluoroindole (5.00 g, 5.00 g, 35.5 mmol, 96 mass percent, 1.00) and Maleimide (1.5 equiv., 5.17 g, 53.3 mmol, 1.50) was charged in a 50 mL vessel, and then Acetonitrile (3 LIkg, 15.0 mL, 11.7 g, 286 mmol, 100 mass percent) and Zinc Chloride (1.05 equiv., 5.08 g, 37.3 mmol, 100 mass percent) were added. The reaction washeated to 85°C over 10 mm and then maintained at 85°C for 24 hrs. While still at 85°C, Water (6 L/kg, 30.0 mL, 30.0 g, 1670 mmol, 100 mass percent) was added slowly, while maintaining the temperature above 80°C. Yellow solids precipitated. The reaction mixture was cooled to 50°C over 1 hour followed by stirring at 50°C for 2 hours, then cooled 10°C over 1 hour. The reaction was stirred at 10°C for 1 hour. The solids werefiltered off, then the filter cake was washed 2 times with 5 ml 1:1 ACN/water to afford isolated compound (6.85 g, 6.85 g, 29.5 mmol, 83.1percent Yield).For purification, the resulting isolated compound was charged (6.85 g, 6.85 g, 29.5mmol, 100 mass percent) into a vessel, followed by addition of Tetrahydrofuran (6 L/kg, 41.1mL, 36.4 g, 505 mmol, 100 mass percent). This mixture was heated to 66°C to form ahomogeneous solution. Heptane (4 L/kg, 27.4 mL, 18.7 g, 187 mmol, 100 mass percent, wasadded slowly at 66°C; solids began to precipitate after 5 volumes. The mixture wascooled to 25°C over 3 hours, then filtered and washed with heptane, followed by dryingin the high vacuum oven overnight. Isolated compound (4.93 g, 4.93 g, 21.2 mmol, 100mass percent, 72.0percent Yield). This isolated compound is Form 2 shown in FIG. 2. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping