|
With pyridine; chromium(VI) oxide; In dichloromethane; at 0 - 20℃; for 2.33333h; |
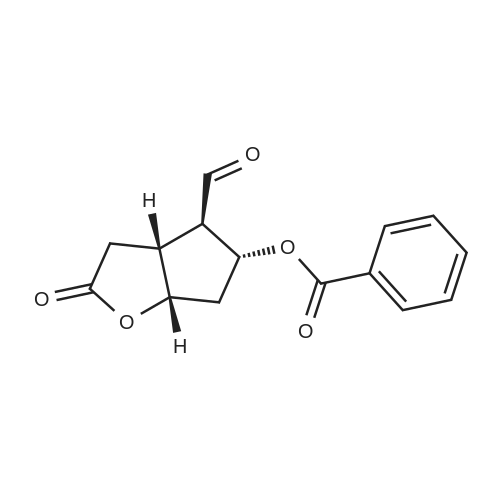
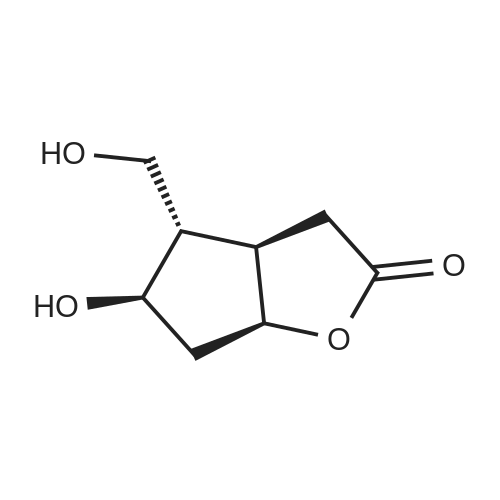
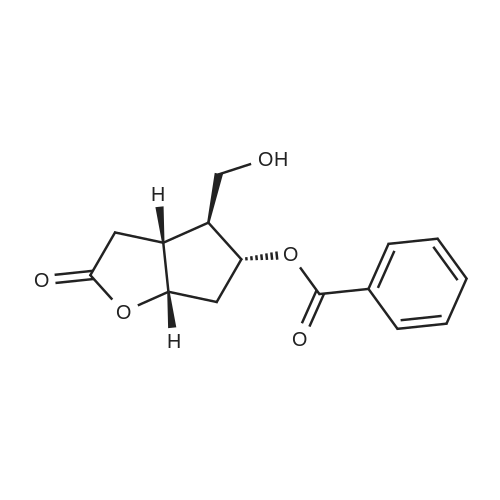
SYNTHETIC PREPARATION 3 Compound of formula (E) A suspension of anhydrous chromium trioxide (20.8 g, 208 mmol) in methylene chloride (400 mL) was stirred and cooled in an ice bath as anhydrous pyridine (32.7 mL, 406 mmol) was added. After 15 min at 0C, the mixture was allowed to warm to ambient temperature for 2h. The reaction mixture was cooled to 0C and treated with a pre-cooled solution of <strong>[39746-00-4](3aR,4S,5R,6aS)-5-(benzoyloxy)hexahydro-4-(hydroxymethyl)-2H-cyclopenta[b]furan-2-one</strong> (Corey lactone, 9.4 g, 34 mmol) in methylene chloride (400mL) After 5 min, the reaction was diluted with toluene (240 mL) and filtered. The solid was washed with toluene. The combined filtrate was concentrated to give (3aR,4R,5R,6aS)-5-(benzoyloxy)hexahydro-2-oxo-2H-cyclopenta[b]furan-4-carboxaldehyde. Toluene was added to give about 300 mL of solution. |
|
With pyridine; chromium(VI) oxide; In dichloromethane; at 0℃; for 0.0833333h; |
Synthetic Preparation 3 Compound of Formula (E) A suspension of anhydrous chromium trioxide (20.8 g, 208 mmol) in methylene chloride (400 mL) was stirred and cooled in an ice bath as anhydrous pyridine (32.7 mL, 406 mmol) was added. After 15 min at 0 C., the mixture was allowed to warm to ambient temperature for 2 h. The reaction mixture was cooled to 0 C. and treated with a pre-cooled solution of <strong>[39746-00-4](3aR,4S,5R,6aS)-5-(benzoyloxy)hexahydro-4-(hydroxymethyl)-2H-cyclopenta[b]furan-2-one</strong> (Corey lactone, 9.4 g, 34 mmol) in methylene chloride (400 mL) After 5 min, the reaction was diluted with toluene (240 mL) and filtered. The solid was washed with toluene. The combined filtrate was concentrated to give (3aR,4R,5R,6aS)-5-(benzoyloxy)hexahydro-2-oxo-2H-cyclopenta[b]furan-4-carboxaldehyde. Toluene was added to give about 300 mL of solution. |
|
|
Example 2: Benzoic acid (3aR, 4R, 5R, 6aS)-2-oxo-4((E)-3-oxo-5-phenyl-pent-1-enyl)-hexahydro-cyclopenta[b]furan-5yl ester (20a, R2= Bz, W = -CH2CH2Ph); To a solution of 14mL of oxalyl chloride (COCl)2 (MW = 126.9; 1.50 eq.) in 300mL of DCM was added a mixture of 29mL of DMSO (MW = 78.1; 3.75 eq.) and 60mL of DCM at -25C. The resulting mixture was stirred for 15min and then a solution of 30g of benzoyl protected Corey alcohol (17a, R2 = Bz; MW = 276.29; 1.0 eq.) in 110mL of DCM was added slowly. Then 84mL of Huenig's base (MW = 129.25; d =0.755; 4.5 eq.) were added and the resulting mixture was stirred for 30min. Then the reaction mixture is poured onto a stirred, pre-cooled (0C) solution of 625mL of water and 20mL of glacial acetic acid (MW = 60.05, d =1.05; 3.2 eq.) and stirred for another 10min at 0C. After separation of the layers the organic layer was washed once with an aqueous NaHCO3 solution (8.6%) and dried by addition of 6.8mL of N,O-bis-trimethylsilylacetamide (MW = 203.43, d = 0.83; 0.25 eq.) at -5C to give 520mL of an organic layer containing benzoyl protected Corey aldehyde (18a, R2 = Bz), which was kept at -5C prior to further processing. |
|
With Dess-Martin periodane; In dichloromethane; at 0℃; for 5h;Inert atmosphere; |
Example 1 Preparation of (3aR,4R,5R,6aS)-4-((E)-4,4-difluoro-3-oxooct-1-enyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate. Dess-Martin periodinane (27.63 g) and dichloromethane (120 mL) are charged into a round bottom flask under nitrogen atmosphere and stirred for 5-10 minutes. The reaction mixture is cooled to 0-5 C. A solution of (3aR,4S, 5R,6aS)-4-(hydroxymethyl)-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate (15 g) in dichloromethane (90 mL) is added to the reaction mixture at 0 C. The reaction mixture is maintained for 5 hours. A solution of sodium thiosulphate pentahydrate (45 g) and sodium bicarbonate (15 g) in water (120 mL) is added to the reaction mixture at 2 C. and maintained for 30 minutes. The reaction mixture is heated to 16 C. and maintained for 30 minutes. Both organic and aqueous layers are separated. The aqueous layer is extracted with dichloromethane (75 mL). The combined organic layer is washed with 10% sodium chloride solution (75 mL). The solvent from the organic layer is evaporated to 10 volumes at 30-35 C. to afford 260 mL of (3aR,4R,5R,6aS)-4-formyl-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate. |
|
With pyridine; dicyclohexyl-carbodiimide; trifluoroacetic acid; In dichloromethane; dimethyl sulfoxide; at 15 - 25℃; for 4h; |
40g of(-)-Corey Lactone Benzoate, 240mlDimethyl sulfoxide , 480ml of Dichloromethane will be added into the reaction flask, add100g(DCC) Dicyclohexylcarbodiimide,stirring and then coolingtothe 15C,add 12 ml Pyridine, 6 ml (TFA)Trifluoroacetic acid, after that stirring at25C till 4h: after stirring add200ml Dimethyl sulfoxide and cool it tothe 15C,thenwe get reaction solution A. Along with the drop of reaction solution A we add consisting 15ml of concentrated hydrochloric acid and 1000mlwater and made the dilute Hydrochloricacid, then stirring 20 min, and filter after the filter we take 100ml dichloromethane for the wash offilter cake, then stratified, water layer extracted with 400ml X 2 dichloromethane,combined with organic layer, use 500ml X 2water for wash, add 100 g anhydrous sodium sulfate for drying dehydration, then filter, after thatwe will get (-)-Corey Lactone Benzoate Aldehyde solution. Along with thissolution add 100g 1-triphenylphosphine-2-heptanone (witting reagent), stirringat 20-30C till 8h forWitting reaction then we will get the reaction solution B. At the 30-40C Dichloromethane distilled under the reduced pressure and add 50ml Anhydrous ethanol evaporated under the reduced pressure. Along with reaction flask add 250ml anhydrous ethanol, andcoolto the -5~-10 C for crystal imitation analysis till 8h then we getcrystalline solution, then filter the solution and take 40 ml X 2 isopropyl ether (0 to 10 C) forthe wash of filter cake then dissolvedthe filtered cake into the 100ml of Dichloromethane and also filter in DCU(1,3-Dicyclohexylurea), after that the filtered solution evaporated under thereduce pressure in 50.3g grease(oil),along with grease(oil) add 101mlanhydrous ethyl alcohol then coolit to the -5~-10 C for static crystalanalysis till 8h,filter it and use appropriate amount of Isopropyl ether andwash cake twice, at 2040C drying at vacuum then get 42.5g needle crystals 15-ketone,yield is 79.2%. |
|
With pyridine; chromium(VI) oxide; In dichloromethane; at 0℃; for 0.0833333h; |
A suspension of anhydrous chromium trioxide (20.8 g, 208 mmol) in methylene chloride (400 mL) was stirred and cooled in an ice bath as anhydrous pyridine (32.7 ml_, 406 mmol) was added. After 15 min at O0C, the mixture was allowed to warm to ambient temperature for 2h. The reaction mixture was cooled to O0C and treated with a pre-cooled solution of (3aR,4S,5R,6aS)-5-(benzoyloxy)hexahydro-4-(hydroxymethyl)-2H- cyclopenta[b]furan-2-one (Corey lactone, 9.4 g, 34 mmol) in methylene chloride (40OmL) After 5 min, the reaction was diluted with toluene (240 mL) and filtered. The solid was washed with toluene. The combined filtrate was concentrated to give (3aR,4R,5R,6aS)-5- (benzoyloxyJhexahydro^-oxo^H-cyclopentalbJfuran-^-carboxaldehyde. Toluene was added to give about 300 mL of solution. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping