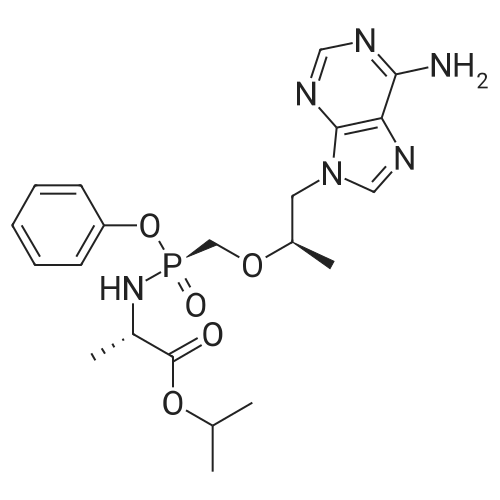
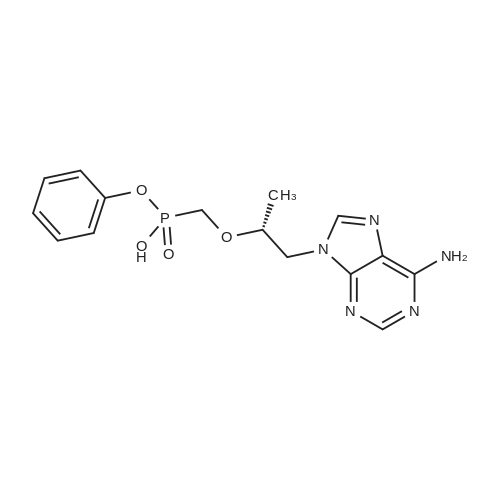
| 190 g |
Stage #1: With thionyl chloride In dichloromethane; toluene at 70 - 80℃; Inert atmosphere
Stage #2: With potassium hydrogencarbonate In dichloromethane; toluene at 15 - 40℃; Inert atmosphere |
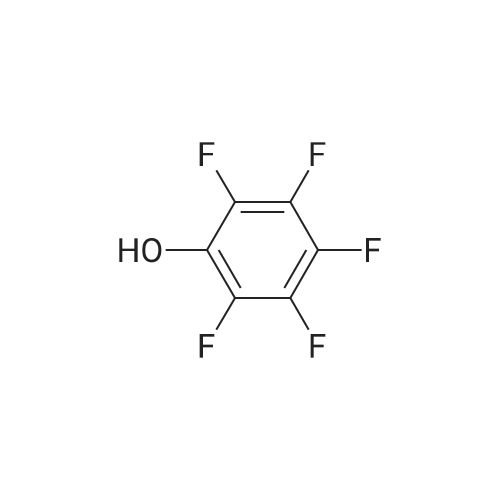
Acrylic chlorination step: 2.4 L of toluene was added to a 5 L reaction flask and TAF-Ml (400 g, prepared according to Example 1) was added,Heated to 70-80°C under nitrogen protection.Slowly add thionyl chloride (262g), add insulation 70-80°C, react for 40-50 hours, temperature below 75°C,Concentrate under reduced pressure until no significant distillate is produced, under nitrogen protection,Cool down to 20-40 °C, add 1600ml of methylene chloride, stir and disperse, wait for use;Dissociating step:Add 2.4L of methylene chloride to the 5L reaction flask, add TAF-SM2 (646g) and potassium bicarbonate (441g) at 15-25°C for 5-10 hours, filter, drain, and control the temperature at 30-40°C. Concentrate dry and press twice with fresh dichloromethane 400 ml*2 and add the residue to fresh dichloromethane 2.4L.Reaction steps:Under nitrogen protection cooling to -25 ~ -15 °C, slowly adding chlorine, adding insulation reaction for 1-2 hours, warming to 20-30 for 1-2 hours.Post-processing:Add 10percent of sodium dihydrogen phosphate aqueous solution 2000g, stir for 5-10 minutes, and stand for layering, the organic phase is respectively used 2000g 10percent sodium dihydrogen phosphate aqueous solution, 2000g*2 5percent potassium hydrogencarbonate aqueous solution, 2000g saturated chlorine The sodium solution and 2000 g of purified water were stirred for 5-10 minutes, and the mixture was allowed to stand for stratification. The organic phase was dried with 250 g-300 g of anhydrous sodium sulfate, filtered, and the mother liquor was dried under reduced pressure to obtain an oily substance under nitrogen protection. Add 400ml of acetonitrile and 1200ml of toluene, warm to 50-60°C, stir for 0.5-1 hour, clear the solution (about 20-30 minutes), slowly cool and crystallize (20-30°C, stir for 1-2 hours to start solidprecipitation) After precipitation of a large amount of solids, cool down to 0-10 °C for 1-2 hours, filter, drain, wash with toluene and dry to obtain crude product.Refining: Add the crude product to the reaction flask, add acetonitrile 1200ml, heat up to 60-70°C under nitrogen protectionSolution, the mother liquor slowly cooled and crystallized, 0-10 °C heat for 1-2 hours, filtered, pumped dry, filter cake 40-50 °C dried under reduced pressure for 10-12 hours to obtain the intermediate 190g TAF-M2. |
| 15 kg |
Stage #1: With thionyl chloride In toluene at 70℃; for 50 h; Reflux; Inert atmosphere; Large scale
Stage #2: With potassium hydrogencarbonate; sodium sulfate In dichloromethane at 20 - 70℃; for 24 h; Inert atmosphere; Large scale
Stage #3: at 20℃; for 2 h; Inert atmosphere; Large scale |
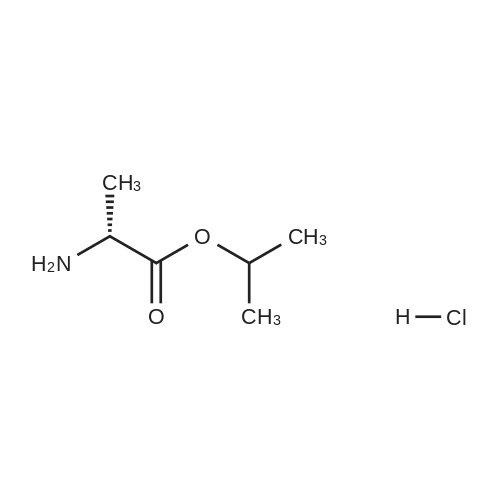
250kg of toluene was pumped into 500L reactor I.Add the above TAF-IM vapor to reflux.The glass return pipe receives water from the bottle and no longer separates the water.After 2 hours of continuous cooling to room temperature,Nitrogen protection,Thionyl chloride 31kg in the high tank,Slowly and repeatedly into the kettle,Leave it at 70°C for 50 hours.Concentrate under reduced pressure to a thick state,After cooling down to room temperature, add 200 kg of n-heptane.Under nitrogen protection filter rejection to dryness,Collect solids,Dissolve complete solids with 200 kg of methylene chloride and transfer to the upper tank.Distill 250kg of dichloromethane into 500L reactor II.Add potassium bicarbonate 50kg,Anhydrous sodium sulfate 10kg and L-alanine isopropyl ester hydrochloride 75kg,Stir for 24 hours under nitrogen protection.Pump down to 500L reactor III.Proline catalyst SA (0.05 eq) was added.The nitrogen protection adds the batches in the high tank to the reactor III in multiple batches.Every time triethylamine 2kg is added,Total added 6kg,After the addition was completed, the mixture was stirred at room temperature for 2 hours.10percent aqueous solution of sodium dihydrogen phosphate in a concentration of 10percentSaturated brine extractionAfter drying over anhydrous sodium sulfate,Suction filtration to 500L dry clean reactorConcentrate under reduced pressure to 60L,Add 100kg of toluene,Then concentrate under reduced pressure to 100L.Then add 100kg of toluene and 50kg of acetonitrile.The solid precipitated slowly at room temperature.The frozen liquid is circulated and incubated for 4 hours.Rejection filter to dry,Acetonitrile beating,Agitate and rinse with acetonitrile.Rejection filter to dry,Drying at 40 °C under reduced pressure to obtain white solid TAF-II M15kg,Yield 45percent (calculated on the basis of "Tenofovir"). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping