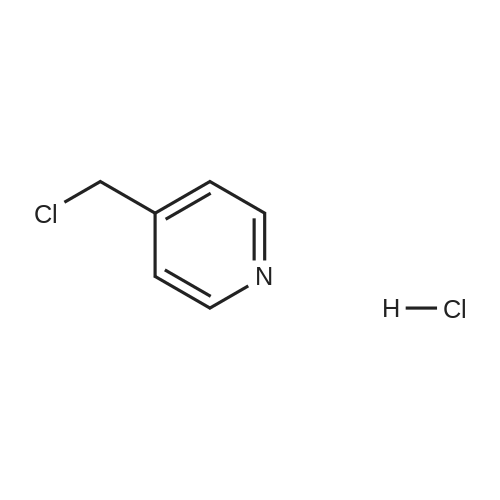
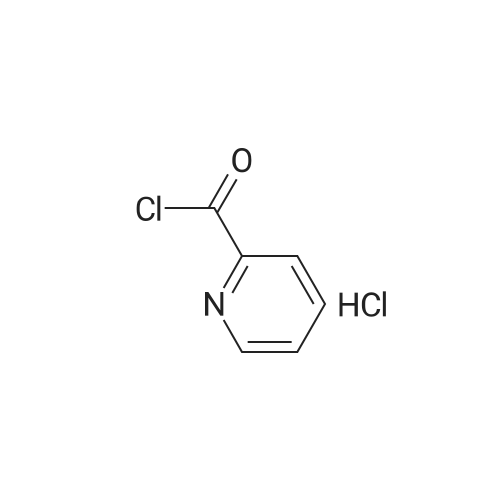
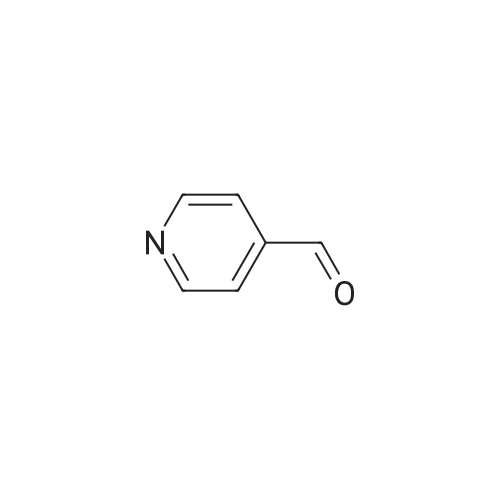
| 86.3% |
With triethylamine; In dichloromethane; at 0℃; for 0.5h;Product distribution / selectivity; |
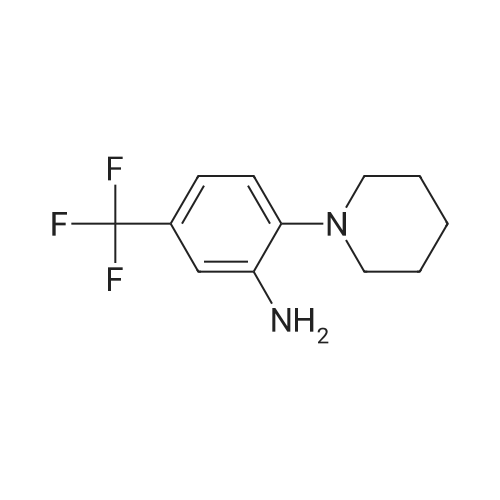
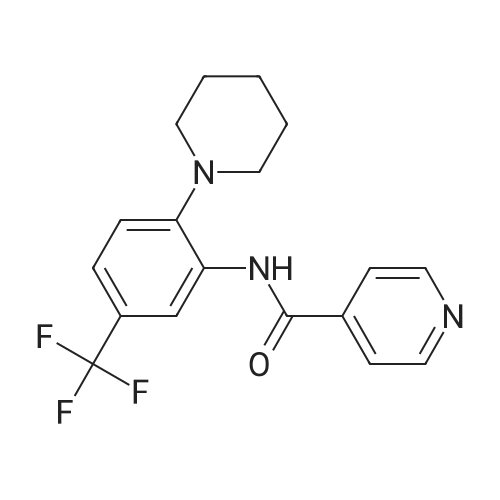
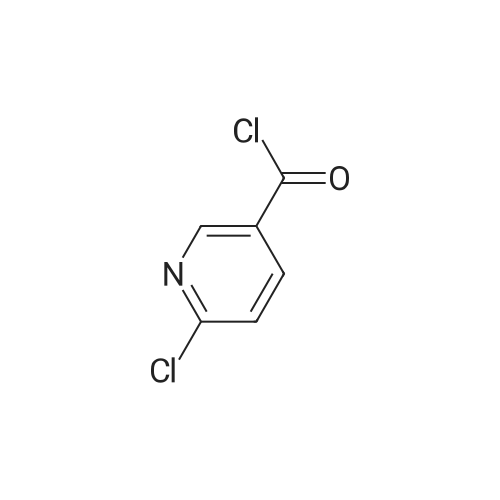
Isonicotinoyl chloride hydrochloride (6.48 g, 36.4 mmol, commercially available product) and triethylamine (5.57 ml, 54.6 mmol) were sequentially added at 0C to a dichloromethane (10 ml) solution of <strong>[1496-40-8]2-(1-piperidinyl)-5-(trifluoromethyl)aniline</strong> (4.45 g, 18.2 mmol) obtained as described in Referential Example 1-2B. The mixture was stirred for half an hour. Water was added to the mixture, and the resulting mixture was extracted three times with ethyl acetate. The obtained organic layer was washed with a saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (200 g, hexane/ethyl acetate = 1/1) and recrystallization. Thus, N-[2-(1-piperidinyl)-5-(trifluoromethyl) phenyl]isonicotinamide (SRPIN-1, GIF-0340) (5.49 g, 86.3%) was yielded as a colorless solid. |
| 75% |
With triethylamine; In dichloromethane; at 0 - 20℃; for 3h; |
A 25 mL round bottom flask initiallyplaced in an ice bath was charged with 0.629 g (3.389 mmol) ofisonicotinoyl chloride hydrochloride, 0.800 mL of triethylamine,8.00 mL of dichoromethane and 0.400 (1.64 mmol) of 2-(piperidin-1-yl)-5-(trifluoromethyl) aniline (8). The ice-bathwas removed andthe mixture was magnetically stirred at room temperature for 3 h.Then, 10.0 mL of distilled water was added, and the mixture wastransferred to a separatory funnel. The aqueous layer was extractedwith ethyl acetate (4 x 30.0 mL). The organic extracts were combinedand the resulting organic layer was washed with brine, driedover sodium sulphate, filtered, and concentrated under reducedpressure. The residue was purified by silica gel column chromatographyeluted with hexane-ethyl acetate (3:1 v/v). The solid wasfurther recrystallized with acetone. The compound SRPIN340 wasobtained as a white solid in 75% yield (430 mg, 1.23 mmol).TLC Rf = 0.13 (hexane - ethyl acetate 3:1 v/v). mp 95.6-96.7 C.IR (ATR, cm-1) numax: 3347, 2945, 2917, 2811, 1679, 1611, 1587, 1556,1527, 1455, 1434, 1380, 1334, 1308, 1239, 1165, 1107, 1093, 1061,1022, 915, 895, 878, 839, 826, 751, 728, 681, 662, 644. 1H NMR(300 MHz, CDCl3) delta: 1.65-1.81 (m, 6H), 2.86 (t, 4H, J = 5.1 Hz), 7.28(d, 1H, J = 8.4 Hz), 7.37 (dd, 1H, J = 8.4 Hz and J = 1.8 Hz), 7.76 (dd,2H, J = 4.5 Hz and J = 1.5 Hz), 8.83-8.85 (m, 3H), 9.55 (s, 1H, NH).13C NMR (75 MHz, CDCl3) delta: 24.0, 27.1, 53.8, 116.6, 120.8, 121.1, 121.6(q, J C-F =3.7 Hz), 124.2 (q, J C-F = 270.5 Hz), 127.5 (q, J C-F = 32.3 Hz),133.4, 141.8, 145.9, 151.1, 163.0. HRMS (M + H+): Calculated forC18H19F3N3O, 350.1480; found: 350.1420. |
| 33.9% |
With dmap; triethylamine; In dichloromethane; at 0 - 20℃; for 19.5h;Product distribution / selectivity; |
Isonicotinoyl chloride hydrochloride (151 mg, 0.850 mmol, commercially available product), triethylamine (450 mul, 3.23 mmol), and a catalytic amount of 4-(dimethylamino)pyridine were sequentially added at 0C to a dichloromethane (5 ml) solution of <strong>[1496-40-8]2-(1-piperidinyl)-5-(trifluoromethyl)aniline</strong> (173 mg, 0.708 mmol), obtained as described in Referential Example 1-2A. The resulting mixture was warmed to room temperature and stirred for 19.5 hours. Water was added to the mixture, and the resulting mixture was extracted three times with ethyl acetate. The obtained organic layer was washed with a saturated aqueous solution of sodium bicarbonate, dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (10 g, hexane/ethyl acetate = 1.5/1) and recrystallization (hexane). Thus, N-[2-(1-piperidinyl)-5-(trifluoromethyl)phenyl]isonicotinamide (SRPIN-1, code name GIF-0340) (83.8 mg, 0.240 mmol, 33.9%) was yielded as a colorless solid. The melting point, and results of TLC and 1H NMR (CDCl3, 400 MHz), are as follows: m.p. 96-98C; TLC Rf 0.40 (hexane/ethyl acetate = 1/1); 1H NMR (CDCl3, 400 MHz) delta 1.67-1.68 (m, 2H, CH2), 1.78 (tt, 4H, J = 5.5, 5.5 Hz, 2CH2), 2.88 (t, 4H, J = 5.5 Hz, 2CH2), 7.29 (d, 1H, J = 8.2 Hz, aromatic), 7.40 (dd, 1H, J = 1.8, 8.2 Hz, aromatic), 7.76 (dd, 2H, J = 2.0, 4.4 Hz, aromatic), 8.86 (dd, 2H, J = 2.0, 4.4 Hz, aromatic), 8.87 (d, 1H, J = 1.8 Hz, aromatic), 9.53 (s, 1H, NH). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +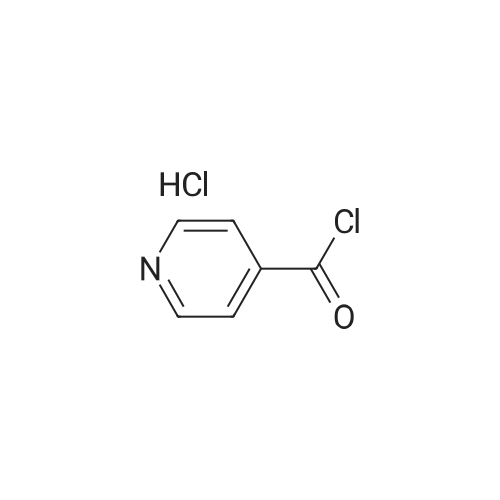

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping