|
|
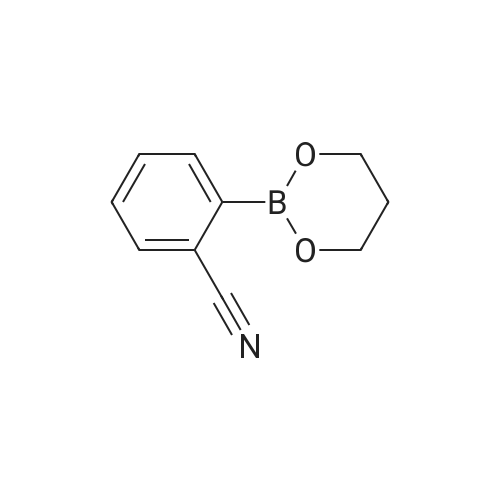
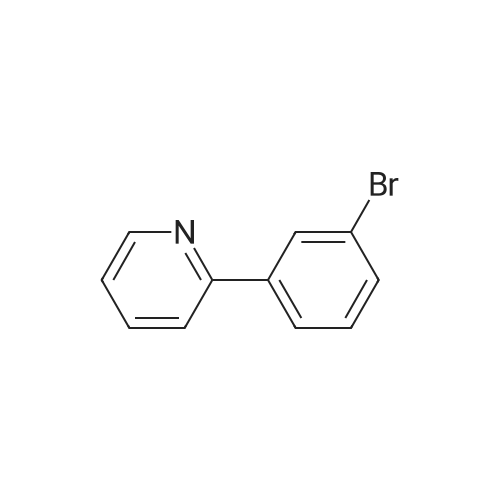
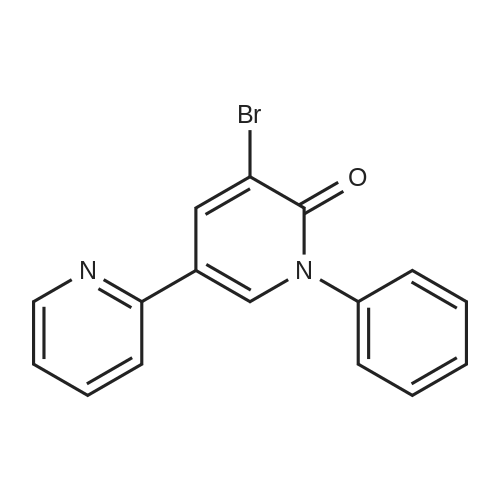
To the reactor containing the whole amount of the crude product of 3-bromo-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one obtained as the residue after concentration in Production Example 2 were added <strong>[172732-52-4]2-(1,3,2-dioxaborinan-2-yl)benzonitrile</strong> (214.9 g), palladium acetate (3.44 g), triphenylphosphine (16.07 g), cuprous iodide (7.29 g), 1,2-dimethoxyethane (3.1 L) and potassium carbonate (158.8 g). Stirring at heating was carried out at 70 °C (external temperature) under a nitrogen atmosphere for 30 minutes and, then, at heating under reflux for 4 hours. Subsequently, ethyl acetate (2.5 L) was added to the reaction mixture at 70 °C (external temperature) and the mixture was stirred for 10 minutes. The reaction mixture was filtrated and the filtrated residue was washed with ethyl acetate (2.5 L). This whole filtrate was transferred to a reactor, to which 12.5percent aqueous ammonia (5 L) was further added. Stirring was carried out at 60 °C (external temperature) for 53 minutes. The lower layer (aqueous layer) in the reaction mixture was separated. 5percent Brine (2.5 L) and 25percent aqueous ammonia (2.5 L) were added to the remaining organic layer. After stirring, the lower (aqueous layer) was separated. 5percent Brine (5 L) was further added to the remaining organic layer. After stirring, the lower (aqueous layer) was separated. The remaining organic layer was concentrated under reduced pressure, and then, acetone (4 L) was added, followed by concentration under reduced pressure. Acetone (7.2 L) and water (0.8 L) were added to this residue, and it was dissolved by stirring at 60 °C (external temperature) for 1 hour and 10 minutes. Next, cooling was carried out at 38 °C (external temperature) for 18 minutes while stirring. To the reaction mixture was added 1 g of seed crystals, crystals of 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one hydrate. Stirring was carried out at 35 °C (external temperature) for 30 minutes. Subsequently, the reaction mixture was stirred at an external temperature being lowered by 5 °C every 30 minutes, and stirred at an external temperature of 10 °C for 17 hours. Water (2.29 L) was added dropwise to the reaction mixture at stirring over a period of 3 hours and 10 minutes. After the addition, stirring continued for additional 1 hour and 20 minutes. The reaction mixture was filtrated and the filtrated residue was washed with 2 L of 50percent acetone-water to give 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one (526.28 g) as a wet cake, which corresponded to 168.3 g as dry weight.Conversion of 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one in the wet cake to dried weight The obtained wet cake (4.378 g) was weighed out and dried under reduced pressure at 50 °C for 4 hours to give 1.4005 g of a dried powder. Converted value as dried weight = (1.4005/4.378) x 526.28 = 168.3 gDetermination of acetone and water weight contents in the wet cake of 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one Gas chromatographic analysis of the obtained wet cake under the conditions described below ascertained that the wet cake obtained in Production Example 3 contained 168 mL of acetone and 186 mL of water.Gas chromatographic analysis conditions: Column: DB-WAX (30m x 0.53mm, 1mum); detector: TCD; oven temp.: 60 °C (8min), 60-180 °C (70 °C/min), 180 °C (5min); detector temp.: 210 °C; inlet temp.: 150 °C; column flow: 5.0 mL/min; split ratio:(1:4); injection vol.: 2 muL |
|
With potassium carbonate;palladium diacetate; copper(l) iodide; triphenylphosphine; In 1,2-dimethoxyethane; at 70℃; for 4.5h;Heating / reflux; |
To the reactor containing the whole amount of the crude product of 3-bromo-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one obtained as the residue after concentration in Production Example 2 were added <strong>[172732-52-4]2-(1,3,2-dioxaborinan-2-yl)benzonitrile</strong> (214.9 g), palladium acetate (3.44 g), triphenylphosphine (16.07 g), cuprous iodide (7.29 g), 1,2-dimethoxyethane (3.1 L) and potassium carbonate (158.8 g). Stirring at heating was carried out at 70° C. (external temperature) under a nitrogen atmosphere for 30 minutes and, then, at heating under reflux for 4 hours.Subsequently, ethyl acetate (2.5 L) was added to the reaction mixture at 70° C. (external temperature) and the mixture was stirred for 10 minutes. The reaction mixture was filtrated and the filtrated residue was washed with ethyl acetate (2.5 L). This whole filtrate was transferred to a reactor, to which 12.5percent aqueous ammonia (5 L) was further added. Stirring was carried out at 60° C. (external temperature) for 53 minutes. The lower layer (aqueous layer) in the reaction mixture was separated. 5percent Brine (2.5 L) and 25percent aqueous ammonia (2.5 L) were added to the remaining organic layer. After stirring, the lower (aqueous layer) was separated. 5percent Brine (5 L) was further added to the remaining organic layer. After stirring, the lower (aqueous layer) was separated. The remaining organic layer was concentrated under reduced pressure, and then, acetone (4 L) was added, followed by concentration under reduced pressure.Acetone (7.2 L) and water (0.8 L) were added to this residue, and it was dissolved by stirring at 60° C. (external temperature) for 1 hour and 10 minutes. Next, cooling was carried out at 38° C. (external temperature) for 18 minutes while stirring. To the reaction mixture was added 1 g of seed crystals, crystals of 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one hydrate. Stirring was carried out at 35° C. (external temperature) for 30 minutes. Subsequently, the reaction mixture was stirred at an external temperature being lowered by 5° C. every 30 minutes, and stirred at an external temperature of 10° C. for 17 hours.Water (2.29 L) was added dropwise to the reaction mixture at stirring over a period of 3 hours and 10 minutes. After the addition, stirring continued for additional 1 hour and 20 minutes. The reaction mixture was filtrated and the filtrated residue was washed with 2 L of 50percent acetone-water to give 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one (526.28 g) as a wet cake, which corresponded to 168.3 g as dry weight. |
|
With copper(l) iodide; potassium carbonate;palladium diacetate; triphenylphosphine; In 1,2-dimethoxyethane; at 70℃; for 4.5h;Heating / reflux; |
(Production Example 3) Synthesis of 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one [Show Image] To the reactor containing the whole amount of the crude product of 3-bromo-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one obtained as the residue after concentration in Production Example 2 were added <strong>[172732-52-4]2-(1,3,2-dioxaborinan-2-yl)benzonitrile</strong> (214.9 g), palladium acetate (3.44 g), triphenylphosphine (16.07 g), cuprous iodide (7.29 g), 1,2-dimethoxyethane (3.1 L) and potassium carbonate (158.8 g). Stirring at heating was carried out at 70 °C (external temperature) under a nitrogen atmosphere for 30 minutes and, then, at heating under reflux for 4 hours. Subsequently, ethyl acetate (2.5 L) was added to the reaction mixture at 70 °C (external temperature) and the mixture was stirred for 10 minutes. The reaction mixture was filtrated and the filtrated residue was washed with ethyl acetate (2.5 L). This whole filtrate was transferred to a reactor, to which 12.5percent aqueous ammonia (5 L) was further added. Stirring was carried out at 60 °C (external temperature) for 53 minutes. The lower layer (aqueous layer) in the reaction mixture was separated. 5percent Brine (2.5 L) and 25percent aqueous ammonia (2.5 L) were added to the remaining organic layer. After stirring, the lower (aqueous layer) was separated. 5percent Brine (5 L) was further added to the remaining organic layer. After stirring, the lower (aqueous layer) was separated. The remaining organic layer was concentrated under reduced pressure, and then, acetone (4 L) was added, followed by concentration under reduced pressure. Acetone (7.2 L) and water (0.8 L) were added to this residue, and it was dissolved by stirring at 60 °C (external temperature) for 1 hour and 10 minutes. Next, cooling was carried out at 38 °C (external temperature) for 18 minutes while stirring. To the reaction mixture was added 1 g of seed crystals, crystals of 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one hydrate. Stirring was carried out at 35 °C (external temperature) for 30 minutes. Subsequently, the reaction mixture was stirred at an external temperature being lowered by 5 °C every 30 minutes, and stirred at an external temperature of 10 °C for 17 hours. Water (2.29 L) was added dropwise to the reaction mixture at stirring over a period of 3 hours and 10 minutes. After the addition, stirring continued for additional 1 hour and 20 minutes. The reaction mixture was filtrated and the filtrated residue was washed with 2 L of 50percent acetone-water to give 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one (526.28 g) as a wet cake, which corresponded to 168.3 g as dry weight. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping