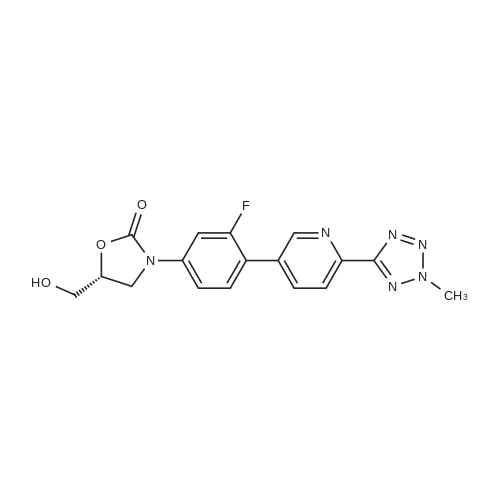
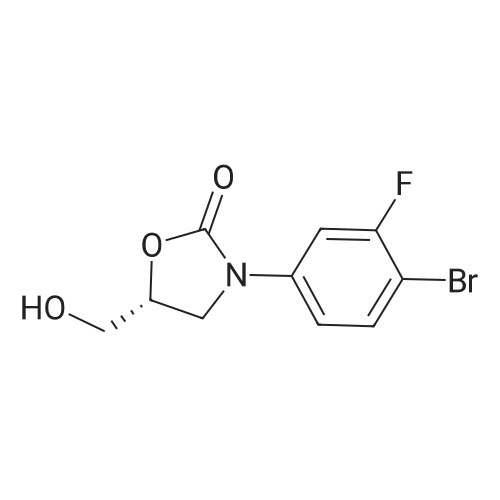
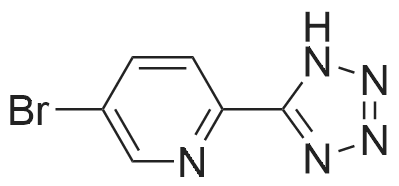
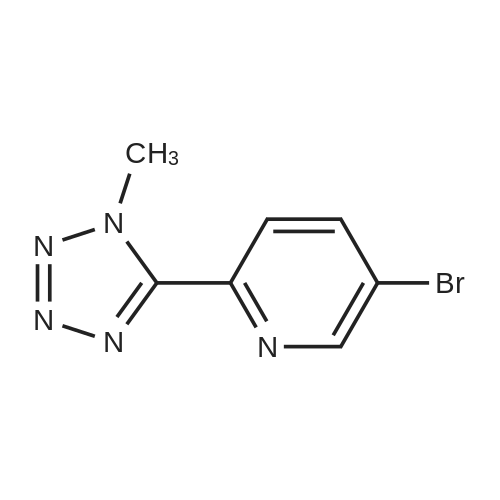
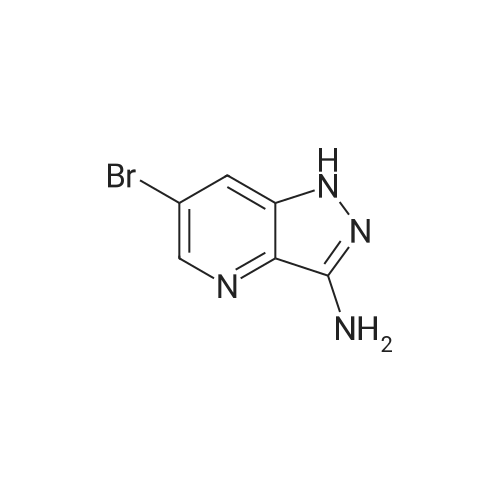
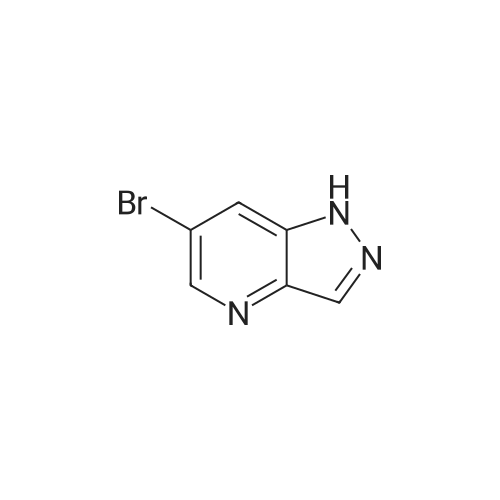
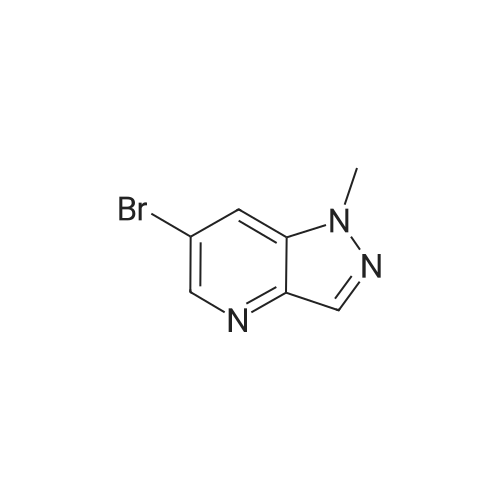
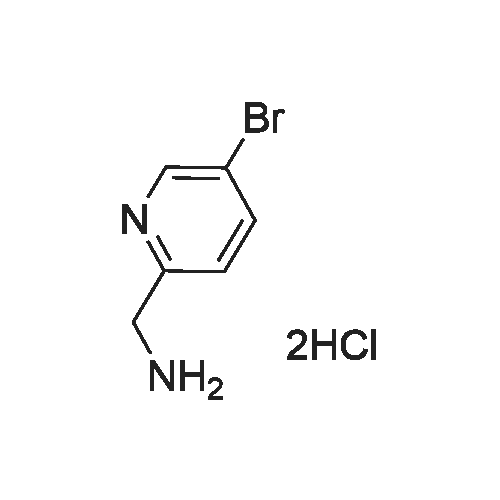
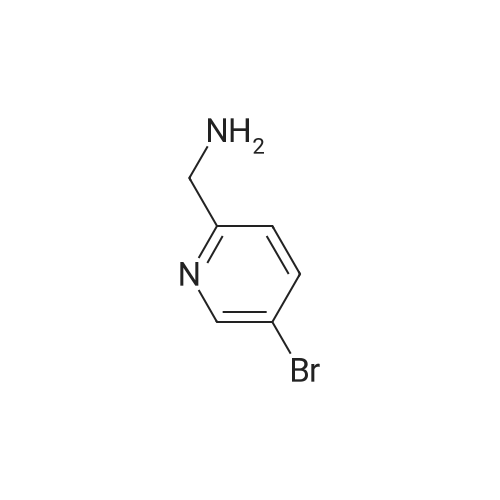
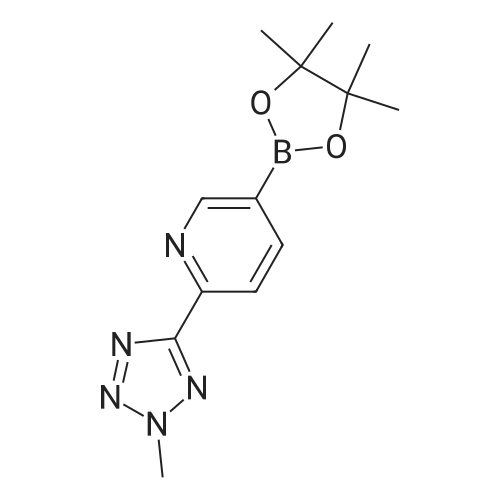
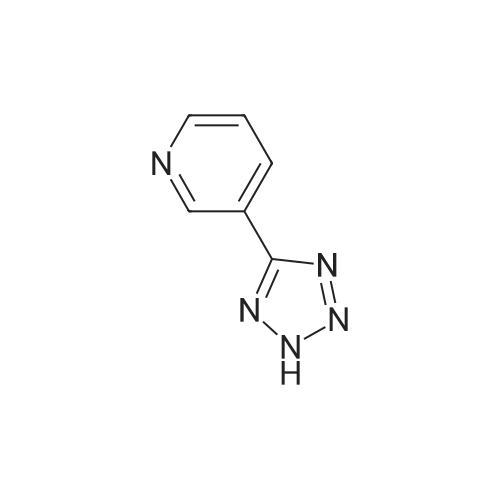
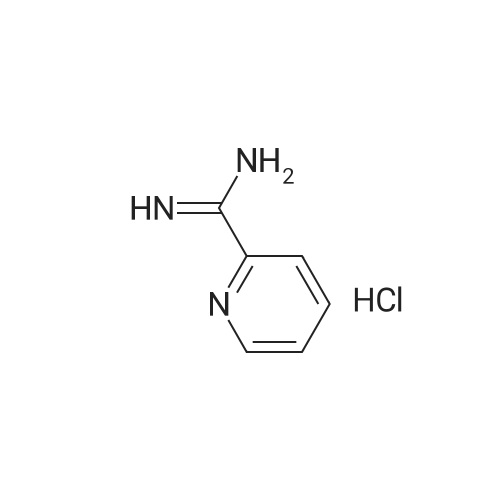
| 48% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; lithium chloride In N,N-dimethyl-formamide at 90℃; for 4 h; |
In a 100 ml reaction flask,Compound 7 (5.0 g, 10 mmol) and 50 ml of DMF were added,2- (1-methyl-tetrazol-5-yl) -5-bromopyridine (2.65, 11 mmol)Lithium chloride (1.7 g),1,1'-bis (diphenylphosphino) ferrocene] palladium chloride (1.0 g),The mixture was stirred at 90 ° C for 4 hours,After completion of the reaction,To room temperature,And extracted three times with water / ethyl acetate (V / V = 1: 1)The organic layers were combined,Dried over anhydrous sodium sulfate,And concentrated to give 1.7 g of the compound (1)The yield was 48percent |
| 26% |
With lithium chloride In 1-methyl-pyrrolidin-2-one at 20 - 120℃; for 4 h; |
In 150ml of 1-methyl-2-pyrrolidone was dissolved 37g of (R)-3- (4- tributhylstannyl-3-fluorophenyl)-2-oxo-5-oxazolidinylmethanol. The solution was added with 19.7g of 2- (2-methyltetrazol-5-yl)-5-bromopyridine, 10.44g of lithium chloride and 2.9g of dichlorobistriphenylphospine palladium (I I) at room temperature and then stirred at the temperature of 120 C for 4 hours. The reaction mixture was added with water and then extracted with ethyl acetate. The organic layer, thus separated, was washed with brine, dehydrated, filtrated, concentrated in vacuo and purified by column chromatography to provide 8g of the title compound. Yield 26percent. 1H NMR (DMSO-d6) 5 8.90 (s, lH), 8. 18 (m, 2H), 7.70 (m, 2H), 7.49 (dd, 1H), 5.25 (t, 1H), 4.74 (m, 1H), 4.46 (s, 3H), 4.14 (t, lH), 3. 88 (dd, lH), 3.68 (m, lH), 3. 58 (m, lH) |
| 26% |
With bis-triphenylphosphine-palladium(II) chloride; lithium chloride In 1-methyl-pyrrolidin-2-one at 120℃; for 4 h; Inert atmosphere |
To a solution of (R)-3-(4-tributhylstannyl-3-fluorophenyl)-2-oxo-5-oxazolidinylmethanol 5 (37.0 g, 74.0 mmol) in NMP (150 mL) was added 2-(2-methyltetrazol-5-yl)-5-bromopyridine 9 (19.7 g, 81.9 mmol), LiCl (10.4 g, 245 mmol) and Pd(PPh3)2Cl2 (2.90 g, 4.13 mmol). The reaction mixture was stirred for 4 h at 120 °C. After being cooled to room temperature, the reaction mixture was poured into water and extracted with EtOAc. The organic layer was washed with brine, dried over anhydrous MgSO4, filtered and concentrated in vacuo. The residue was further purified by column chromatography to obtain the title compound (8 g, 26percent). Mp: 201 °C. 1H NMR (DMSO-d6): δ 8.90 (s, 1H), 8.18 (m, 2H), 7.70 (m, 2H), 7.49 (dd, J = 8.8 Hz, 2.4 Hz, 1H), 5.25 (t, J = 5.6 Hz, 1H), 4.74 (m, 1H), 4.46 (s, 3H), 4.14 (t, J = 8.8 Hz, 1H), 3.88 (m, 1H), 3.68 (m, 1H), 3.58 (m, 1H). 13C NMR (DMSO-d6): δ 163.56, 159.03, 154.06, 149.18, 144.78, 140.30, 136.96, 131.42, 130.73, 121.89, 118.36, 113.81, 105.18, 73.39, 61.52, 45.96, 39.63. IR (neat, cm-1): 3251.40, 1746.23, 1619.91, 1473.35, 1406.82. [M + H]+: 371.02. HRMS [EI-MS]: m/z, calculated for [C17H15FN6O3] 370.1190, found, 370.1100 [C17H15FN6O3]. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +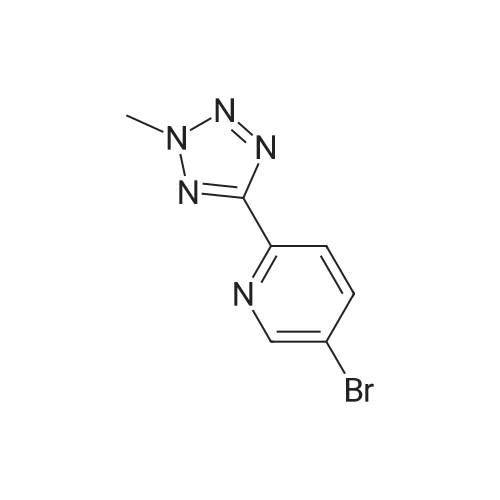

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping