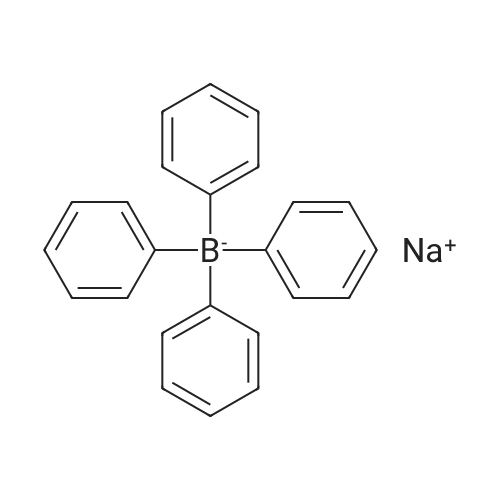
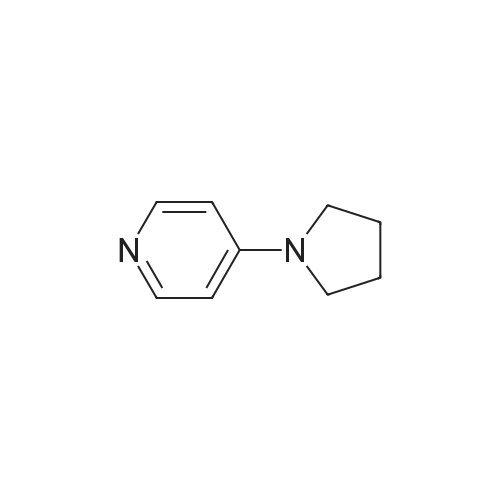
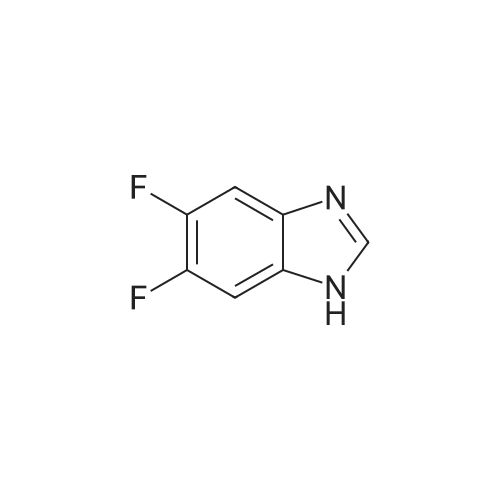
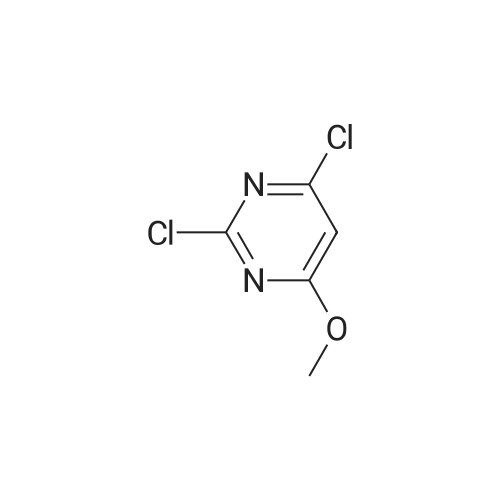
| 64% |
|
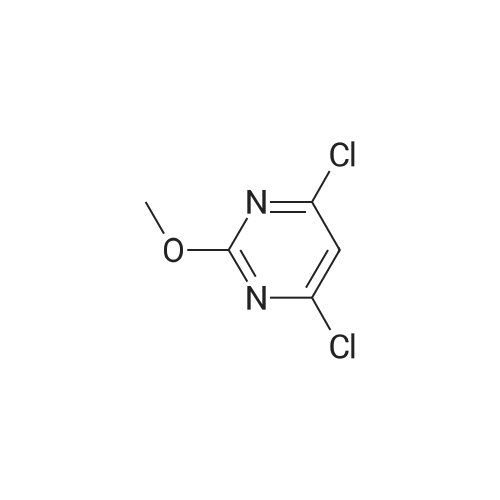
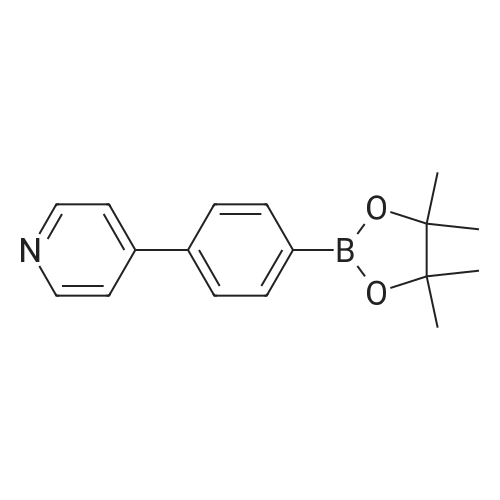
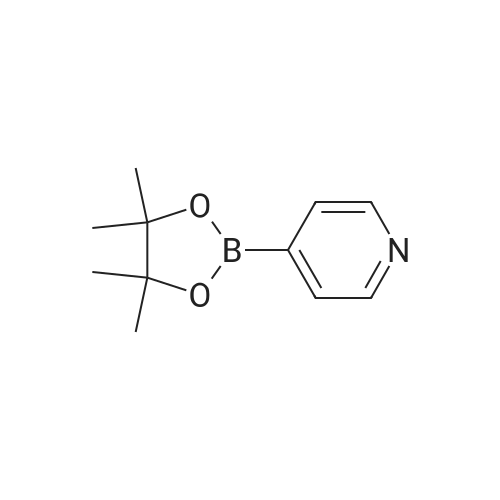
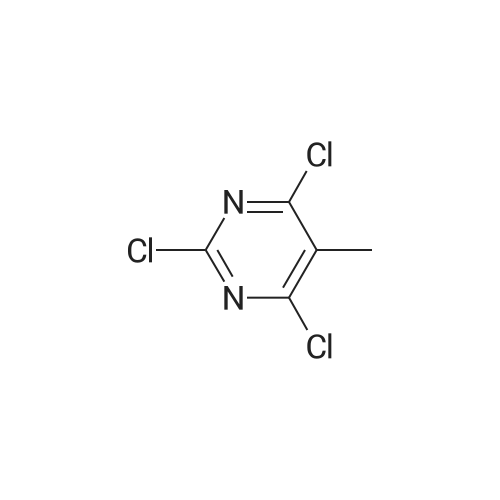
<a> Synthesis of Intermediate (80- 1) Intermediate (80" l) A flask was charged with 2,4,6-trichloropyridine (732 mg, 4.0 mmol), followed by being purged with argon gas. To the flask, thereafter, dioxane (60 niL), which had been deaerated with argon gas, 4-(4-pyridyl)phenyl boronic acid pinacol ester (8.0 mmol, 2.26 g), and bis(triphenylphosphine)palladium(II) dichloride (0.3 mmol, 210 mg) were added. After bubbling the solution with argon gas, 2M K2CO3 (20 mL) was added, and the resultant was heated and stirred at 50C for 4 hours. Next, 4-4,4, 5,5-tetramethyl- l,3,2-dioxaborolan-2-yl)pyridine (4 mmol, 820 mg) was added and the resultant was heated and stirred at 100C for 4 hours. Subsequently, the resultant was filtered with Celite, and water and chloroform were added to the filtrate to separate an organic layer. Thereafter, a water layer was extracted 5 times with chloroform. The combined organic layer was washed with saturated salt water, followed by drying with sodium sulfate to condensate the filtrate, to thereby obtain a crude product. The crude product was purified by silica-gel column chromatography (eluent: chloroform/methanol = 93/7), and the obtained solids were dispersed and washed in chloroform/hexane. The solids were collected by filtration, and the obtained solids were vacuum dried to thereby obtain an intermediate (80- 1) as pale yellow solids (the yielded amount: 1.19 g, the yield: 64% H NMR (500 MHz, CDCI3, delta) : 8.87 (dd, Ji = 4.7 Hz, J2 = 1.7 Hz, 2H), 8.75 (dd, Ji = 4.0 Hz, J2 = 1.7 Hz, 4H), 8.58 (dd, Ji = 4.7 Hz, J2 = 1.7 Hz, 2H), 8.45 (dt Ji = 8.0 Hz, J2 = 1.7 Hz, 4H), 8.21 (s, 1H), 7.88 (dd, Ji = 8.6 Hz, J2 = 1.7 Hz, 4H), 7.61 (dd, Ji = 4.7 Hz, J2 = 1.7 Hz, 4H) |
| 61% |
|
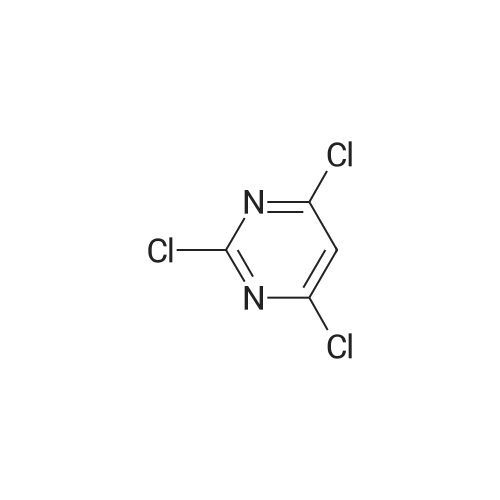
2, 4, 6-trichloropyrimidine (732 mg, 4.0 mmol) was placed in a flask,After substitution with argon gas,Dioxane (60 mL) degassed with argon gas,4- (4-pyridyl) phenylboronic acid pinacol ester (8.0 mmol, 2.26 g) represented by the following structural formula (28)Bis (triphenylphosphine) palladium (II) dichloride (0.3 mmol, 210 mg) was added.After bubbling the solution with argon gas,2 M K 2 CO 3 (20 mL) was added,And the mixture was heated and stirred at 50 C. for 4 hours.Then 4- (4,4,5,5-tetramethyl-1,3,2-dioxeSabololan-2-yl) pyridine(4 mmol, 820 mg) was added,And the mixture was heated and stirred at 100 C. for 4 hours.Subsequently, the contents were filtered through celite, water and chloroform were added to the filtrate to separate the organic layer,The aqueous layer was extracted with chloroform.The combined organic layer was dried over sodium sulfate and the filtrate was concentrated to give a crude product.This was purified by silica gel chromatography (developing solvent: chloroform / methanol)The solid obtained after the concentration was dried under vacuum,The target compound was obtained as a pale yellow solid (yield 1.13 g, yield 61%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping