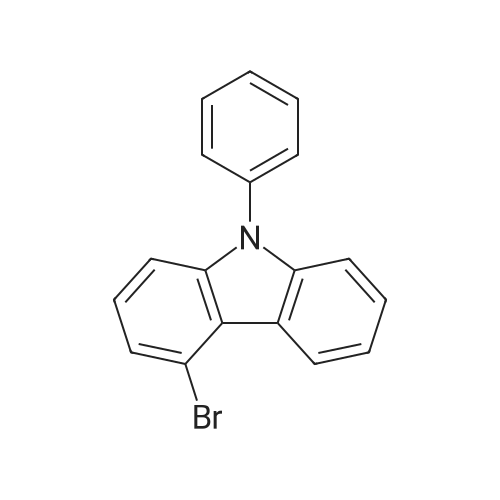
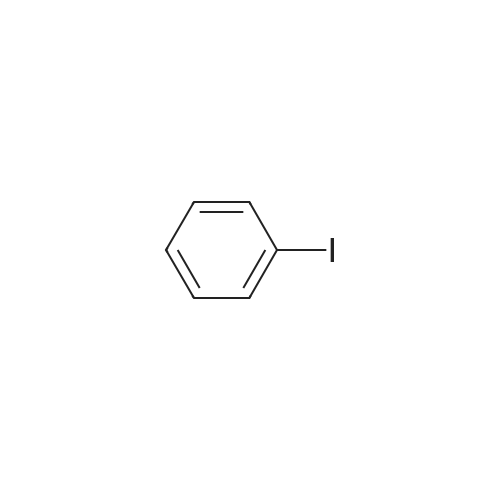
| 89% |
With 1,10-Phenanthroline; 18-crown-6 ether; potassium carbonate; copper(l) chloride; In 1-methyl-pyrrolidin-2-one; at 180℃;Inert atmosphere; |
Under nitrogen protection, 4-bromocarbazole (20g, 81.3mmol),Iodobenzene (99.5g, 487.6mmol),Cuprous chloride (4.0g, 40.6mmol),Potassium carbonate (168.5g, 1218.9mmol), 18-crown-6 (10.7g, 40.6mmol),1,10-Phenanthroline (7.3g, 40.6mmol) and N-methylpyrrolidone (508mL) were added to a three-necked flask and reacted at 180 C overnight. After cooling to room temperature, distilled water was added, the mixture was extracted with dichloromethane, the organic phase was washed with water, the organic phase was dried over anhydrous sodium sulfate and concentrated to remove the solvent, and the residue was purified by column chromatography (pure petroleum ether) to give beige oily intermediate B 116g (yield: 89%). |
| 88% |
With 18-crown-6 ether; copper; potassium carbonate; In 1,2-dichloro-benzene; at 180℃; for 24h; |
4-bromo-9H-carbazole 20.0 g (81.3 mmol), 2-iodobenzene 33.2 g (162.5 mmol), copper powder 10.3 g (162.5 mmol), 18-crown-6 4.3 g (16.3 mmol), 33.7 g (243.9 mmol) of potassium carbonate was added in order, 200 ml of 1,2-dichlorobenzene was added, and the mixture was refluxed and stirred at 180 C. for 1 day. When the reaction was completed, the solution was concentrated under reduced pressure after a high temperature filter, and the organic layer solution was subjected to column chromatography with hexane to obtain 21.0 g of [Intermediate 1-e]. (Yield 88%) |
| 88% |
With 18-crown-6 ether; copper; potassium carbonate; In 1,2-dichloro-benzene; at 180℃; for 24h; |
4-bromo-9H-carbazole 20.0 g (81.3 mmol), 2-iodobenzene 33.2 g (162.5 mmol),Copper powder 10.3 g (162.5 mmol), 18-crown-6 4.3 g (16.3 mmol), potassium carbonate 33.7 g (243.9 mmol) were added in order,After adding 200 ml of 1,2-dichlorobenzene, the mixture was refluxed and stirred at 180 C. for 1 day.When the reaction was completed, the solution was concentrated under reduced pressure after high-temperature filtering, and the organic layer solution was subjected to column chromatography with hexane to obtain 21.0 g of [Intermediate 5-b]. (Yield 88%) |
| 83% |
With copper(l) iodide; 1,10-Phenanthroline; potassium carbonate; In N,N-dimethyl-formamide; for 24h;Inert atmosphere; Reflux; |
A 5 L flask was charged with 200.0 g (0.8 mol) of intermediate 4-bromo-9H-carbazole,248.7 g (1.2 mol) of iodobenzene,168.5 g (1.2 mol) of potassium carbonate,31.0 g (0.2 mol) of copper iodide (CuI) 29.3 g (0.2 mol) of 1,10-phenanthroline was added to 2.5 L of N, N-dimethylformamide,And refluxed under a nitrogen stream for 24 hours.The resulting mixture was added to 4 L of distilled water, and the crystallized solid was filtered, washed with water, methanol and hexane. The obtained solid was extracted with water and dichloromethane, and the obtained water was removed from the obtained organic layer using magnesium sulfate. The filtrate was concentrated and purified by column chromatography to obtain Intermediate C-1 as a white solid (216.2 g, 83% yield) . |
| 83% |
With copper(l) iodide; 1,10-Phenanthroline; potassium carbonate; In N,N-dimethyl-formamide; for 24h;Reflux; Inert atmosphere; |
In a 5 L flask, Bromo-9H-carbazole 200.0 g (0.8 mol),248.7 g (1.2 mol) of iodobenzene,168.5 g (1.2 mol) of potassium carbonate,31.0 g (0.2 mol) of copper (I) iodide,29.3 g (0.2 mol) of 1,10-phenanthrolineAfter adding 2.5 L of N, N-dimethylformamide,And refluxed under a nitrogen stream for 24 hours.The resulting mixture was added to 4 L of distilled water, and the crystallized solid was filtered, washed with water, methanol and hexane.The obtained solid was extracted with water and dichloromethane, and water was removed from the obtained organic layer using magnesium sulfate and concentrated,Purification by column chromatography gave Intermediate C-1 as a white solid(216.2 g, 83% yield). |
| 83% |
With copper(l) iodide; 1,10-Phenanthroline; potassium carbonate; In N,N-dimethyl-formamide; for 24h;Inert atmosphere; Reflux; |
A 5 L flask was charged with 200.0 g (0.8 mol) of intermediate 4-bromo-9H-carbazole, 248.7 g (1.2 mol) of iodobenzene, 168.5 g (1.2 mol) of potassium carbonate, 31.0 g (0.2 mol) of copper (I) iodide and 29.3 g (0.2 mol) of 1,10-phenanthroline were added to 2.5 L of N, N-dimethylformamide, And refluxed under a nitrogen stream for 24 hours. The resulting mixture was added to 4 L of distilled water and the crystallized solid was filtered, Water and methanol,Washed with hexane. The obtained solid was extracted with water and dichloromethane, and the obtained aqueous layer was extracted with magnesium sulfate. The filtrate was concentrated and purified by column chromatography to obtain Intermediate I-1 as a white solid (216.2 g, 83% yield) . |
| 83% |
With copper(l) iodide; 1,10-Phenanthroline; potassium carbonate; In N,N-dimethyl-formamide; for 24h;Inert atmosphere; Reflux; |
A 5 L flask was charged with 200.0 g (0.8 mol) of intermediate 4-bromo-9H-carbazole,248.7 g (1.2 mol) of iodobenzene,168.5 g (1.2 mol) of potassium carbonate,31.0 g (0.2 mol) of copper (I) iodide,29.3 g (0.2 mol) of 1,10-phenanthroline,Was added to 2.5 L of N, N-dimethylformamide,And refluxed under a nitrogen stream for 24 hours.The resulting mixture was added to 4 L of distilled water, and the crystallized solid was filtered, washed with water, methanol and hexane. The obtained solid was extracted with water and dichloromethane, and the obtained aqueous layer was extracted with magnesium sulfate. The filtrate was concentrated and purified by column chromatography to obtain Intermediate I-1 as a white solid (216.2 g, 83% yield) . |
| 83% |
With copper(l) iodide; 1,10-Phenanthroline; potassium carbonate; In N,N-dimethyl-formamide; for 24h;Reflux; Inert atmosphere; |
200.0 g (0.8 mol) of an intermediate of 4-bromo-9H-carbazole, 248.7 g (1.2 mol) of iodo benzene, 168.5 g (1.2 mol) of potassium carbonate, 31.0 g (0.2 mol) of copper(I) iodide, and 29.3 g (0.2 mol) of 1,10-phenanthroline were mixed with 2.5 L of N,N-dimethylformamide in a 5 L flask, and the mixture was refluxed under a nitrogen flow for 24 hours. The obtained mixture was added to 4 L of distilled water, and a solid crystallized therein was filtered and washed with water, methanol, and hexane. Subsequently, the solid was extracted with water and dichloromethane, and an organic layer obtained therefrom was treated by using magnesium sulfate to remove moisture and then, concentrated and purified through column chromatography to obtain Intermediate I-1 as a white solid (216.2 g, a yield of 83%). |
| 81% |
With copper(l) iodide; 1,10-Phenanthroline; potassium carbonate; In N,N-dimethyl-formamide; at 80℃; for 24h;Inert atmosphere; |
[287] In a 100 ml round-bottom three-neck flask under a nitrogen atmosphere, 2.6 g of Intermediate 24, 2.6 g of iodobenzene, 0.2 g of copper iodide, 0.2 g of1, 10-phenanthroline, 4.4 g of potassium carbonate and 30 ml of dimethylfomiamide were placed, and reacted at 80C for 24 hrs. The reaction solution was cooled and then extracted with dichloromethane and water. The extracted solution was concentrated, subjected to column chromatography using a solvent mixture of dichloromethane and n-hexane and then concentrated, thus obtaining 2.7 g of Intermediate 25 (yield 8 1%).[288] MS (ESI): [M+Hj 322 |
| 75% |
With tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate; In toluene; at 100℃; |
Reaction is progressed in 100C after putting the sub 6-4-4 (4.9g, 20mmol), sub 6-4-5 (4.1g, 20mmol), pd2(dba)3 (0.9g, 1 mmol), PPh3 (0.5g, 2mmol), NaOt-Bu (5.8g, 60mmol), toluene (210mL) in the round bottom flask. If reaction was completed the organic compound which was dry to the MgSO4 and was the organic layer generated after doing the concentration was re-determined as ether and water after doing the extraction with the silicagel column and the Sub 6-4-6 was obtained with 4.8g (yield : 75%). |
| 75% |
With tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate; In toluene; at 100℃; |
To a round bottom flask Sub 1-1-4-1 (4.9g, 20mmol), Sub 1-1-5-1 (4.1g, 20mmol), Pd2 (dba) 3 (0.9g, 1mmol), PPh3 (0.5g , 2mmol), and NaOt-Bu (5.8g, 60mmol), the reaction proceeds at 100 C after loading into toluene (210mL). After the reaction was completed, the organic layer was dried over MgSO4 and the ether was extracted with water and the resulting organics concentrated to silica gel column and recrystallized the Sub 1-1-6-1 4.8g (yield: 75%) was obtained. |
| 73% |
With 18-crown-6 ether; copper; potassium carbonate; In N,N-dimethyl-formamide; at 190℃; for 12h;Inert atmosphere; |
A 1000 mL 2-neck round bottom flask was charged with intermediate 6-2 (100.0 mmol, 24.6 g), Iodobenzene (120.0 mmol, 24.5 g),Cu-metal (110.0 mmol, 7.0 g), K2CO3 (150.0 mmol, 20.7 g),18-Crown-6 (10.0 mmol, 2.64 g) was charged and charged with nitrogen. 500 mL of dimethylformamide was added as a solvent, and the mixture was refluxed at 190 DEG C for 12 hours.The temperature of the reaction solution was lowered to room temperature and extracted with water and diethyl ether. The obtained extract was dried over MgSO4 and dried under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel column chromatography to obtain 23.5 g (yield: 73%) of Intermediate 6-3 as a yellow solid. |
| 68% |
|
4-bromo-9Hcarbazole(2 g, 8 mmol), copper(Ι) iodide (1.24 g, 6.5 mmol), cesiumcarbonate (7.98 g, 24 mmol) were added to 25 mL of anhydrous toluenesolution in 3-neck round bottom flask under N2 atmosphere. After themixture was heated at 80 C, iodobenzene (1.82 mL, 16 mmol), ethylenediamine(0.82 mL, 12.2 mmol) was added to mixture. Then themixture was refluxed at 110 C for 15 h (1.7 g, Yield 68%) 1H NMR(300 MHz, THF): δ(ppm) 8.81-8.78 (d, 1H), 7.65-7.59 (t, 2H),7.56-7.47 (m, 3H), 7.45-7.40 (m, 2H), 7.35-7.27 (q, 3H), 7.25-7.20 (t,1H). |
|
With tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate; In toluene; at 100℃; for 24h; |
2-bromo-9H-carbazole ( 327mmol) and iodobenzene (360mmol), toluene (2800mL) Pd2(dba) 3 (8.24g, 9mmol), PPh3 (7.87g, 30mmol), NaOt-Bu ( 86.5g, 900mmol) were added to each, and refluxed under stirring for 24 hours at 100 C. ether and extracted with water and the organic layer is MgSO 4 and then dried, and concentrated to the resulting organic silicagel column and recrystallization to give the 2-bromo-9-phenyl- 9H-carbazole. |
|
With 18-crown-6 ether; copper; potassium carbonate; In N,N-dimethyl-formamide; at 190℃; for 12h;Inert atmosphere; |
A 1000 mL 2-neck round bottom flask was charged with intermediate 6-2 (100.0 mmol, 24.6 g), Iodobenzene (120.0 mmol, 24.5 g), Cu metal (110.0 mmol, 7.0 g), K2CO3 (150.0 mmol, 20.7 g), 18-Crown-6 (10.0 mmol, 2.64 g) was charged and charged with nitrogen. To this was added 500 mL of dimethylformamide as a solvent And then refluxed at 190 C for 12 hours. The temperature of the reaction solution was lowered to room temperature and extracted with water and diethyl ether. The obtained extract was dried over MgSO4 and dried under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel column chromatography to obtain 23.5 g (yield: 73%) of Intermediate 6-3 as a yellow solid |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping