| 71% |
With sodium carbonate In 1,4-dioxane; water; acetonitrile at 100℃; for 4 h; Inert atmosphere |
Step 7:
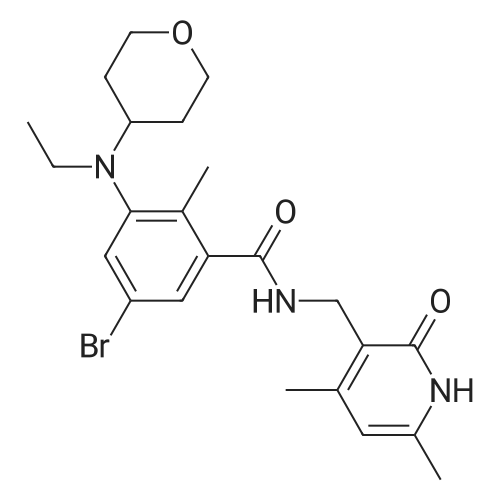
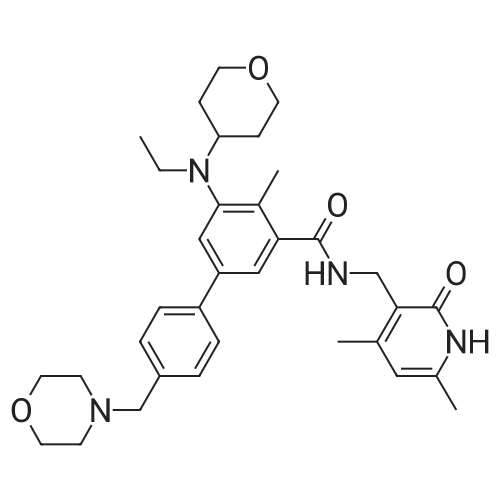
Synthesis of N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide
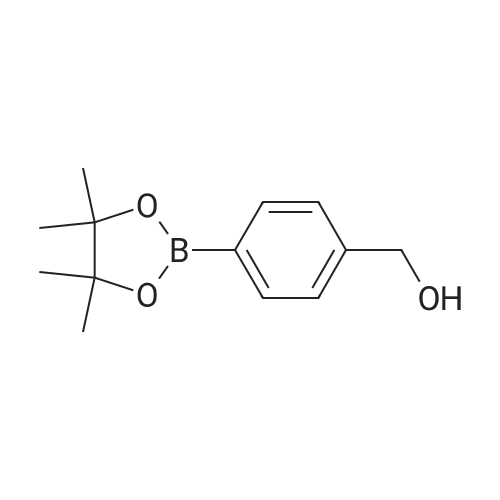
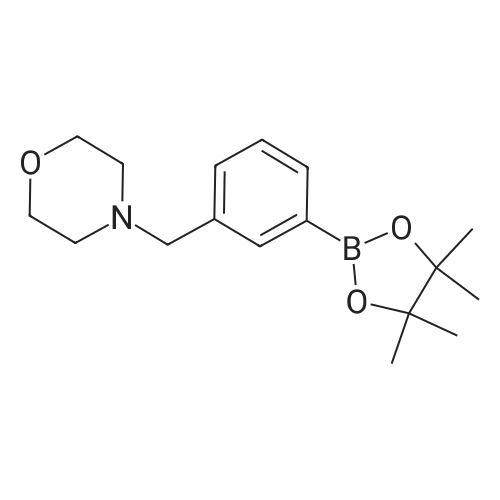
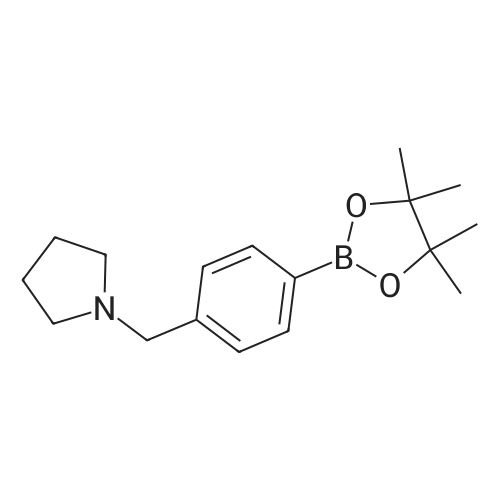
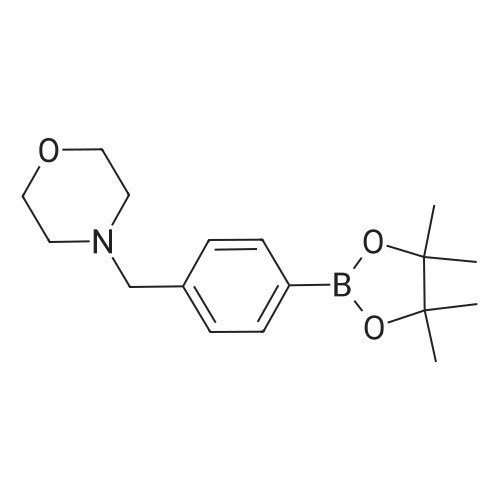
To a stirred solution of 5-bromo-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-2-methylbenzamide (14 g, 29.5 mmol) in dioxane/water mixture (70 mL/14 mL) was added 4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl)morpholine (13.4 g, 44.2 mmol) followed by addition of Na2CO3 (11.2 g, 106.1 mmol).
The solution was purged with argon for 15 minutes and then Pd (PPh3)4 (3.40 g, 2.94 mmol) was added and the solution was again purged with argon for a further 10 min.
The reaction mixture was heated at 100° C. for 4 h.
After completion (monitored by TLC), the reaction mixture was diluted with water and extracted with 10percent MeOH/DCM.
The combined organic layers were dried over anhydrous sodium sulphate, filtered and concentrated under reduced pressure.
The crude compound was purified by column chromatography (100-200 mesh silica gel) eluting with methanol: DCM to the title compound as a solid (12 g, 71percent).
Analytical Data: LCMS: 573.35 (M+1)+; HPLC: 99.5percent ( 254 nm) (Rt; 3.999; Method: Column: YMC ODS-A 150 mm*4.6 mm*5μ; Mobile Phase: A; 0.05percent TFA in water/B; 0.05percent TFA in acetonitrile; Inj. Vol: 10 μL, Col. Temp.: 30° C.; Flow rate: 1.4 mL/min.;
Gradient: 5percent B to 95percent B in 8 min, Hold for 1.5 min, 9.51-12 min 5percent B); 1H NMR (DMSO-d6, 400 MHz) δ 11.46 (s, 1H), 8.19 (t, 1H), 7.57 (d, 2H, J=7.2 Hz), 7.36-7.39 (m, 3H), 7.21 (s, 1H), 5.85 (s, 1H), 4.28 (d, 2H, J=2.8 Hz), 3.82 (d, 2H, J=9.6 Hz), 3.57 (bs, 4H), 3.48 (s, 2H), 3.24 (t, 2H, J=10.8 Hz), 3.07-3.09 (m, 2H), 3.01 (m, 1H), 2.36 (m, 4H), 2.24 (s, 3H), 2.20 (s, 3H), 2.10 (s, 3H), 1.64-1.67 (m, 2H), 1.51-1.53 (m, 2H), 0.83 (t, 3H, J=6.4 Hz). |
| 71% |
Stage #1: With sodium carbonate In 1,4-dioxane; water for 0.25 h; Inert atmosphere
Stage #2: With tetrakis(triphenylphosphine) palladium(0) In 1,4-dioxane at 100℃; for 4.2 h; Inert atmosphere |
[0336] Step 7: Synthesis of N-((4, 6-dimethyl-2-oxo-l, 2-dihydropyridin-3-yl) methyl)-5- (ethyl (tetrahydro-2H-pyran-4-yl) amino)-4-methyl-4'-(morpholinomethyl)-[l, l'-biphenyl]-3- carboxamide [0337] To a stirred solution of 5-bromo-N-((4, 6-dimethyl-2-oxo-l, 2-dihydropyridin-3-yl) methyl)-3-(ethyl (tetrahydro-2H-pyran-4-yl) amino)-2-methylbenzamide (14 g, 29.5 mmol) in dioxane/ water mixture (70 mL/14 mL) was added 4-(4-(4, 4, 5, 5-tetramethyl-l, 3, 2- dioxaborolan-2-yl) benzyl) morpholine (13.4 g, 44.2 mmol) followed by addition of Na2C03 (11.2 g, 106.1 mmol). The solution was purged with argon for 15 minutes and then Pd (PPh )4 (3.40 g, 2.94 mmol) was added and the solution was again purged with argon for a further 10 min. The reaction mixture was heated at 100°C for 4 h. After completion (monitored by TLC), the reaction mixture was diluted with water and extracted with 10percent MeOH/DCM. The combined organic layers were dried over anhydrous sodium sulphate, filtered and concentrated under reduced pressure. The crude compound was purified by column chromatography (100- 200 mesh silica gel) eluting with methanol: DCM to the title compound as a solid (12 g, 71 percent). Analytical Data: LCMS: 573.35 (M + 1)+; HPLC: 99.5percent ( 254 nm) (R,;3.999; Method: Column: YMC ODS-A 150 mm x 4.6 mm x 5 μ; Mobile Phase: A; 0.05percent TFA in water/ B; 0.05percent TFA in acetonitrile; Inj. Vol: 10 μ, Col. Temp.: 30 °C; Flow rate: 1.4 mL/min.; Gradient: 5percent B to 95percent B in 8 min, Hold for 1.5 min, 9.51-12 min 5percent B); 1H NMR (DMSO-J6, 400 MHz) δ 11.46 (s, 1H), 8.19 (t, 1H), 7.57 (d, 2H, J=7.2 Hz), 7.36-7.39 (m, 3H), 7.21 (s, 1H), 5.85 (s, 1H), 4.28 (d, 2H, J=2.8 Hz), 3.82 (d, 2H, J=9.6 Hz), 3.57 (bs, 4H), 3.48 (s, 2H), 3.24 (t, 2H, J=10.8Hz), 3.07-3.09 (m, 2H), 3.01 (m, 1H), 2.36 (m, 4H), 2.24 (s, 3H), 2.20 (s, 3H), 2.10 (s, 3H), 1.64-1.67 (m, 2H), 1.51-1.53 (m, 2H), 0.83 (t, 3H, J=6.4 Hz). |
| 71% |
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In 1,4-dioxane; water at 100℃; for 4 h; Inert atmosphere |
N - [(4,6- dimethyl-2-carboxy-1,2-dihydro-3-pyridyl) methyl] -2-methyl-3- [ethyl (tetrahydro -2H- 4- pyran-yl) amino] -5-carboxamide bromobenzene (14g, 29.5mmol) was dissolved in dioxane / water mixture (70mL /14mL), added with stirring 4- (4-morpholino-methyl) phenylboronic acid pinacol ester (13.4g, 44.2mmol), followed by the addition of sodium carbonate (11.2g, 105.7mmol). Solution was purged with argon for 15min, then add tetrakis (triphenylphosphine) palladium (3.40g, 2.94mmol), a solution of purified argon and then 10min. The reaction mixture was heated to 100 reaction 4h. Tracking progress of the reaction TLC, eluent: methanol / dichloromethane (1:10), add water, 50mL, methanol / dichloromethane: extraction (19). The organic layer was dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was chromatographed on silica gel column, eluent: methanol / dichloromethane (1:10) to give a solid product 12g, Yield 71percent |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping