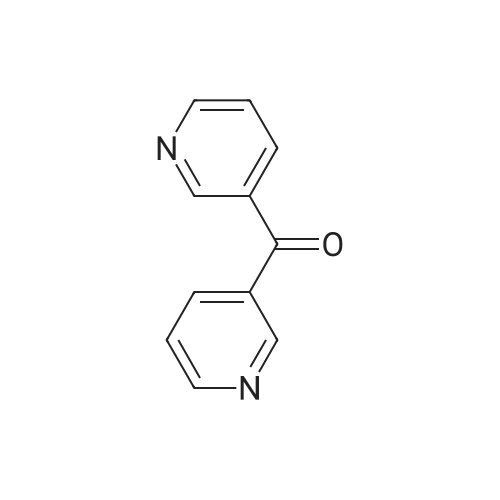
| 91% |
With potassium phosphate;palladium(II) acetylacetonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; In 1-methyl-pyrrolidin-2-one; at 100℃; for 18h;Inert atmosphere;Product distribution / selectivity; |
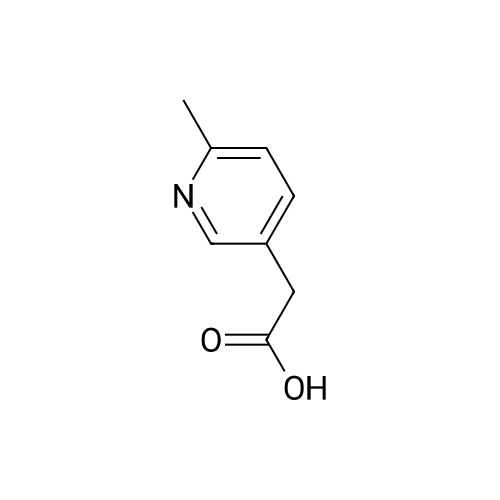
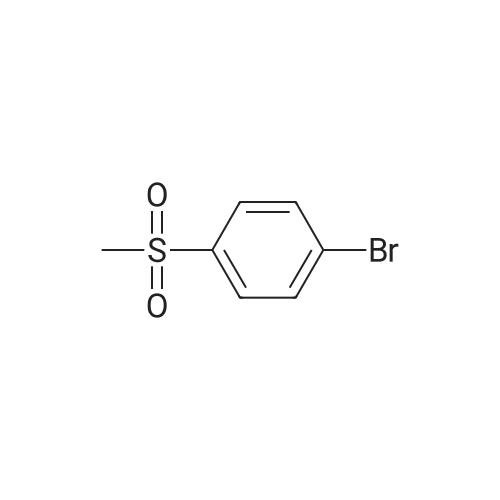
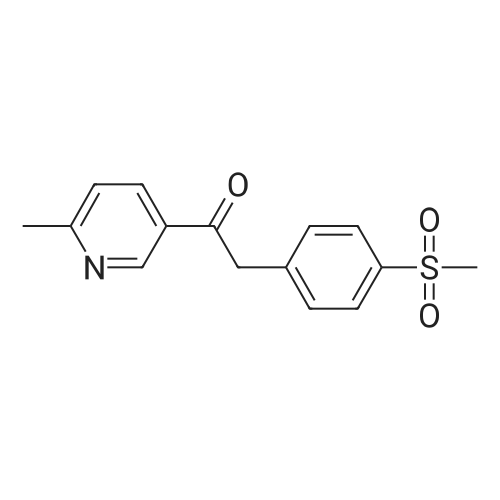
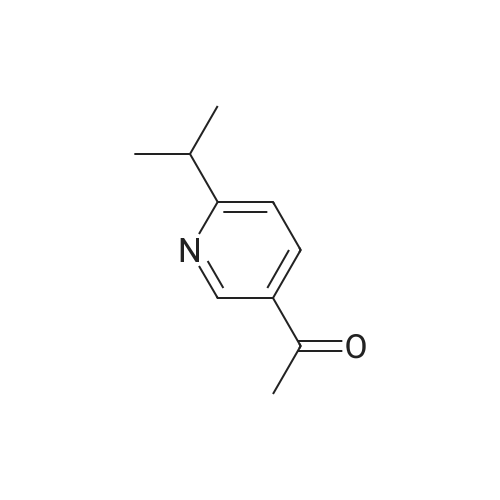
Pd(acac)2 (6.1 mg, 0.02 mmol, 0.5 mol %) and Xantphos (23.2 mg, 0.04 mmol, 1 mol o) are introduced into a flared flask provided with coolant. 4-bromophenylmethylsulfone of formula (III, XBr) (1.17 g, 5 mmol), acetylpicoline of formula (II) (541 mg, 4 mmol) and K3PO4 (2.55 g, 12.0 mmol, 3 eq) are added thereto. Once the argon atmosphere has been stabilized with vacuum-argon cycles, anhydrous and degassed NMP (15 ml) is added with a syringe. The mixture is then kept stirred under stirring in an argon atmosphere for 18 h at 100 C. The conversion is quantitative. The reaction mixture is diluted with a saturated solution of NaHCO3 (50 mL) and extracted with AcOEt (4×50 mL). The combined organic phases were washed with an aqueous solution saturated with NaHCO3 (30 mL), anhydrified on MgSO4 and concentrated in a vacuum. The residue was purified by silica gel chromatography using AcOEt/cyclohexane as eluent in a gradient from 5:5 to 10:0. 1.05 g product were obtained, for a molar yield of 91% as a white crystalline solid. |
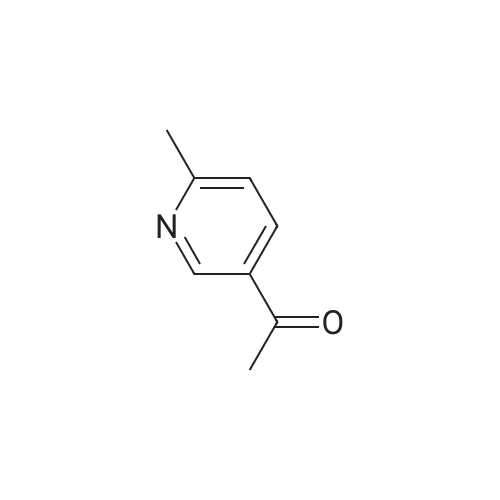
| 75% |
With tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; sodium t-butanolate; In toluene; for 5h;Inert atmosphere; Reflux; |
Xantphos 0.00267 g (0.0046 mmol) and Pd2(dba)3 0.00177 g (0.0031 mmol) in 15 ml of anhydrous toluene were charged in a reactor under inert atmosphere. 4-bromophenyl methyl sulfone 0.724 g (3.078 mmol) and 3-acetyl-6-methyl pyridine 0.416 g (3.079 mmol) were then added. The mixture was heated to reflux and a suspension of t-BuONa 0.71 g in 15 ml of anhydrous toluene was added dropwise over about 4 h. After about 1 h from completion of the addition, the reaction mixture was cooled to 20 C and a solution of diluted hydrochloric acid to acidic pH was added. The aqueous phase was separated and added dropwise over 1 h to a mixture of water 8.3 g, ethyl acetate 15.3 g and sodium bicarbonate 2.1 g at 60 C. At addition completed and after maintaining the temperature at 60 C for 1 h, it was checked that the pH was between 4 and 7, the mixture was cooled to 20 C filtered and dried under vacuum at 50 C. 0.67 g of the compound of formula 1 was obtained with a yield of 75 %. |
| 75% |
With tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; sodium t-butanolate; In toluene; for 4h;Inert atmosphere; Reflux; |
Xantphos 0.00267 g (0.0046 mmol) and Pd2(dba)3 0.00177 g (0.0031 mmol) in 15 ml of anhydrous toluene were charged in a reactor under inert atmosphere. 4-bromophenyl methyl sulfone 0.724 g (3.078 mmol) and 3-acetyl-6-methyl pyridine 0.416 g (3.079 mmol) were then added. The mixture was heated to reflux and a suspension of t-BuONa 0.71 g in 15 ml of anhydrous toluene was added dropwise over about 4 h. After about 1 h from completion of the addition, the reaction mixture was cooled to 20C and a solution of diluted hydrochloric acid to acidic pH was added. The aqueous phase was separated and added dropwise over 1 h to a mixture of water 8.3 g, ethyl acetate 15.3 g and sodium bicarbonate 2.1 g at 60C. At addition completed and after maintaining the temperature at 60C for 1 h, it was checked that the pH was between 4 and 7, the mixture was cooled to 20C filtered and dried under vacuum at 50 C. 0.67 g of the compound of formula 1 was obtained with a yield of 75 %. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping