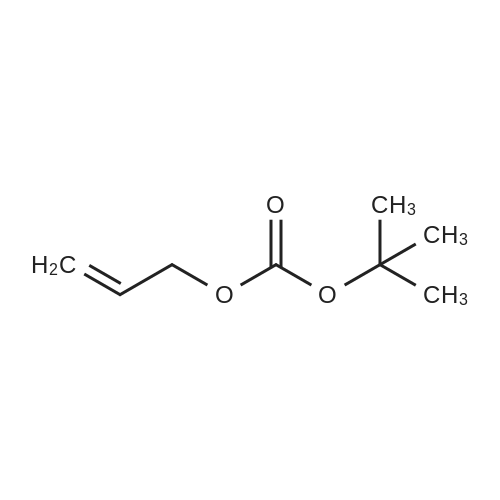
|
With triphenylphosphine;5%-palladium/activated carbon; at 77.5 - 80℃; for 10.1167h; |
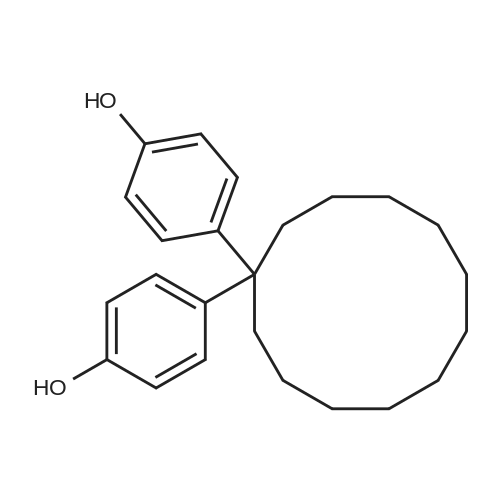
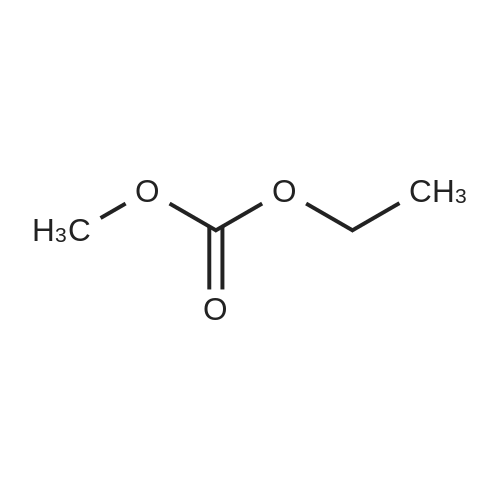
Allyl alcohol (101.58 grams, 1.75 moles), dimethyl carbonate (157.55 grams, 1.75 moles) and sodium methoxide catalyst (0.18 gram, 0.065 percent by weight) were added to a 500 milliliter, 3 neck, round bottom glass reactor and maintained at room temperature (23C) with stirring under a nitrogen atmosphere. The reactor was additionally outfitted with a chilled condenser, a thermometer, magnetic stirring, and a thermostatically controlled heating mantle. An equilibrium mixture of allylmethyl carbonate, diallyl carbonate and methanol was rapidly formed concurrent with cooling of the reactor contents to 15.5C. After 13 minutes l,l-bis(4-hydroxyphenyl)cyclododecane (28.31 grams, 0.1606 equivalent of hydroxy groups) was added to the reactor, followed by a mixture of triphenylphosphine (0.56 gram, 0.204 percent by weight) and 5% palladium on carbon (0.38 gram, 0.127 percent by weight). The l,l-bis(4-hydroxyphenyl)cyclododecane assayed 99.76 area % via high pressure liquid chromatographic (HPLC) analysis with the balance consisting of 2 minor components (0.09 and 0.15 area %). Heating commenced and over the next 127 minutes the reaction temperature reached 79 - 800C. The reaction mixture was maintained <n="37"/>for 8 hours at 77.5 - 800C and then cooled to room temperature and vacuum filtered through a bed of diatomaceous earth packed on a medium fritted glass funnel. The recovered filtrate was rotary evaporated at a maximum oil bath temperature of 1000C and to a vacuum of 1.7 mm Hg pressure to provide a transparent, light yellow colored, liquid (35.04 grams) which became a tacky solid at room temperature.[0119] HPLC analysis revealed the presence of 96.78 area % allyl ether of l,l-bis(4- hydroxyphenyl)cyclododecane with the balance as a single minor component (3.22 area %). The single minor component was removed by dissolving the product in dichloromethane (100 milliliters) and passing the resultant solution through a 2 inch deep by 1.75 inch diameter bed of silica gel (230-400 mesh particle size, 60 angstrom mean pore size, 550 m2/gram surface dimension) supported on a medium fritted glass funnel. After elution from the silica gel bed with additional dichloromethane, a yellow band remained in the region of the origin. Rotary evaporation provided 33.98 grams (98.94 % isolated yield) of pale yellow colored tacky solid.[0120] HPLC analysis revealed the presence of 99.57 area % allyl ether of l,l-bis(4- hydroxyphenyl)cyclododecane with the balance as 2 minor components (0.22 and 0.21 area %). Infrared spectrophotometric analysis of a film sample of the product on a KBr plate revealed peaks in the range expected for unsaturated C-H stretch (3032, 3058, 3081 cm" 1X saturated C-H stretch (2862, 2934 cm"1 [shoulder present on both]), C=C stretch (1581, 1607 cm"1), C-O stretch (1026 cm"1), and CH=CH2 deformation (924, 998 cm"1), accompanied by total absence of hydroxyl group absorbance thus confirming full conversion of the phenolic hydroxyl groups to allyl ether groups. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping