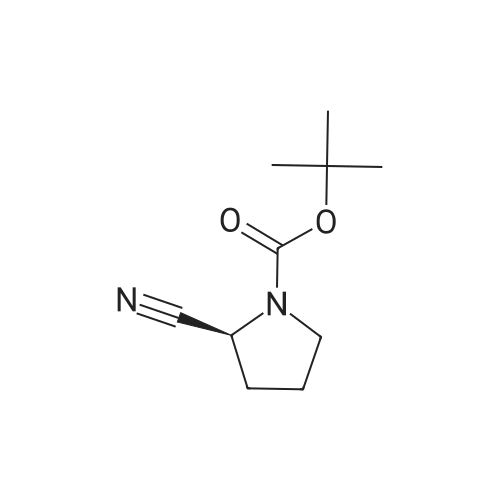
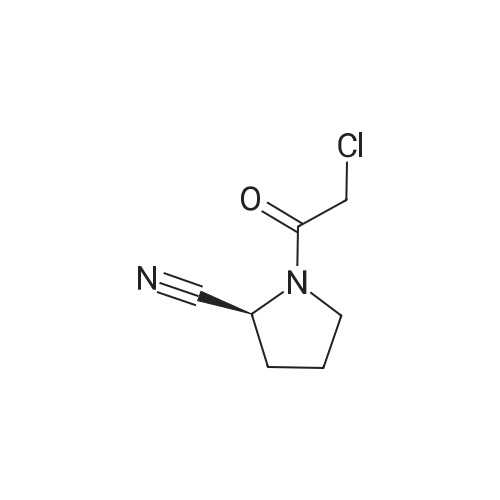
| 76% |
With 1,3,5-trichloro-2,4,6-triazine; In N,N-dimethyl-formamide; at 20℃; for 1h; |
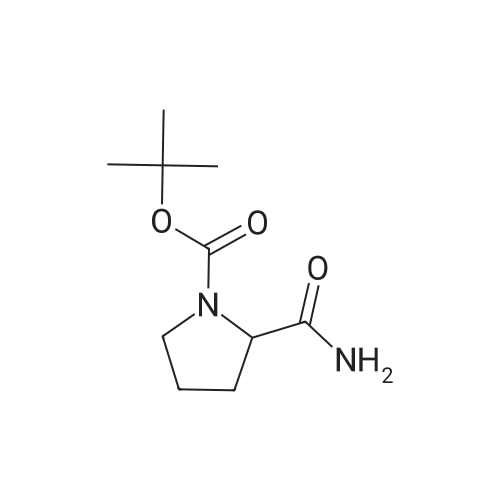
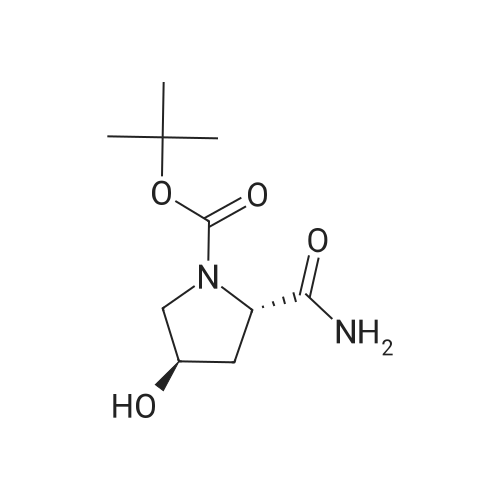
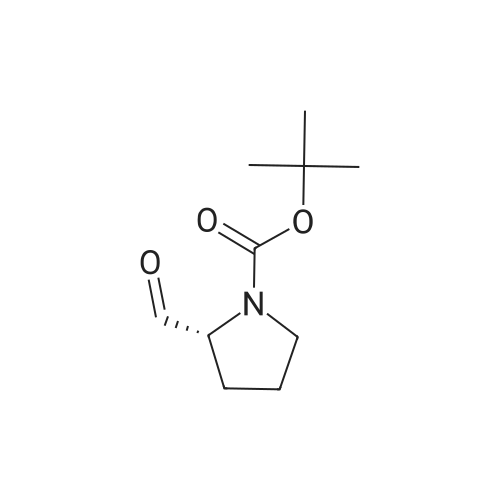
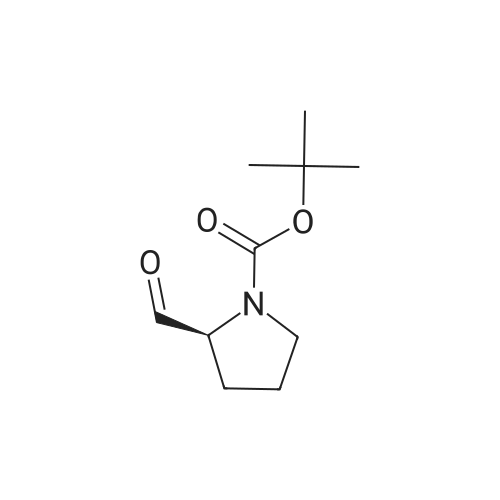
General procedure: A mixture of compound 2 (5 g, 23.3 mmol) and cyanuric chiloride (2.58 g, 14.0 mmol) in DMF (10 mL) was stirred at room temperature for 1 h (monitored by TLC). After the reaction completed, the solution was extracted with EtOAc, washed, dried, concentrated, and purified by flash chromatography on silica gel, eluted with a mixture of PE/EA (1/1, v/v), to afford 3 (3.48 g, 76%) as a white solid. 1H NMR (300 MHz, CDCl3) delta 4.76 (s, 1H), 3.51 (s, 2H), 2.34-2.31 (m, 1H), 2.17-2.10 (m, 2H), 1.90-1.87 (m, 1H), 1.49 (s, 9H). MS (ESI) m/z 197 [M+H]+. |
| 76% |
With 1,3,5-trichloro-2,4,6-triazine; In N,N-dimethyl-formamide; at 20℃; for 1h; |
A mixture of compound 9 (5 g, 23.3 mmol) and cyanuric chiloride (2.58 g, 14.0 mmol) in DMF (10 mL) was stirred at roomtemperature for 1 h (monitored by TLC). After the reaction completed, thesolution was extracted with EtOAc, washed, dried, concentrated, and purified byflash chromatography on silica gel, eluted with a mixture of PE/EA (1/1, v/v)to afford 10 (3.48 g, 76%) as a white solid. 1H NMR (CDCl3,300 MHz): delta4.76(s, 1H), 3.51 (s, 2H), 2.34-2.31 (m, 1H), 2.17-2.10 (m, 2H), 1.90-1.87 (m, 1H),1.49 (s, 9H). MS (ESI) m/z 197[M+H]+. |
|
With 1,3,5-trichloro-2,4,6-triazine; N,N-dimethyl-formamide; In dichloromethane; at 20 - 40℃;Inert atmosphere; |
Dichloromethane (500 ml), L-Prolinamide (100 g), potassium carbonate (60.50 g) were mixed and stirred under nitrogen atmosphere in round bottom flask The reaction mass was cooled to 10-15C and then Di-tert-butyl dicarbonate (210.30 g) was added. Reaction mixture was stirred till completion of the reaction. After completion water (500m1) was added and product was extracted in dichloromethane. Dichloro methane was removed and then dimethyl formamide (140 ml) and dichloromethane (700 ml) were added under nitrogen atmosphere. Cyanuric chloride (72.70 g) was charged in 2-3 equal lots to the reaction mass at 20-25C under nitrogen atmosphere. The reaction mass was maintained at 35-40C for 4-5 hrs. After completion of the reaction solid was filtered and washed with MDC. Methane sulfonic acid (252.60 g) was added to the filtrate and heated the reaction mass to 40-45C for 3-4 hrs. After completion of the reaction the reaction mass was cooled at 0-5C and Triethyl amine (88.65 g) and Chloroacetyl chloride (118.70 g) were added. The reaction mass was maintained at 20-30C for 1-2 hrs under nitrogen atmosphere. After completion of the reaction water was charged and product was extracted in dichloromethane. Organic layer was washed first with dilute HC1 solution and then with ammonia solution. Organic solvent was removed and product was crystallized using isopropyl alcohol. Yield: 75.0gm |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping