| 95% |
|
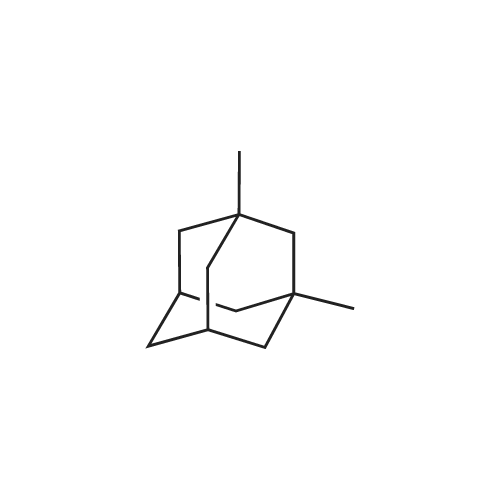
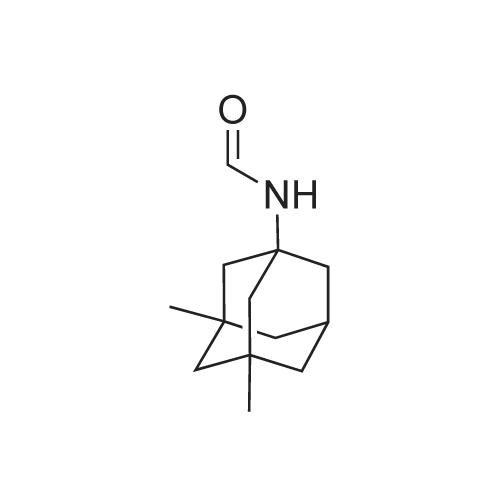
Example 1 300 g (1.83 mol) of <strong>[702-79-4]1,3-dimethyladamantane</strong> (<strong>[702-79-4]1,3-DMA</strong>) and 900 ml of sulfuric acid 96% (16.2 mol) are mixed and cooled to 0C to 5C. 84 ml (1.20 mol) of nitric acid 65% are added under vigorous stirring over a time period of 4.5 hours, keeping the temperature between 0C to 5C. Stirring is continued overnight at that temperature. This solution is concurrently added together with 180 ml of formamide (4.53 mol) into a vessel precharged with sulfuric acid 96% (150 ml) preheated to 55 - 60C, over the time period of two hours, keeping the internal temperature at T = 55C - 60C. After completion of the formylation, the mixture is cooled to 5C and quenched into a cold solution (0C), containing water (720 ml), aqueous ammonia 30% (480 ml) and dichloromethane (900 ml), while keeping the temperature below 25C. The aqueous acidic layer is re-extracted with 450 ml of dichloromethane. The combined organic layers are washed three times with 600 ml water. With the last washing the pH is corrected to 8 to 9 using aqueous ammonia. N-Formyl-1-amino-3,5-dimethyladamantane is obtained as a solution in dichloromethane, which can be used in the subsequent step. Solvent removal gives 360 g of N-Formyl-1-amino-3,5-dimethyladamantane (NFORM) (yield 95%). The purity as measured by GC is 98%. |
| 89.3% |
|
EXAMPLE 1 Synthesis of 1-formamido-3,5-dimethyladamantane In sequence, 4 mL 65% technical nitric acid and then within three hours 50 mL 98% technical sulfuric acid are added to 6.572 g (40 mmol) <strong>[702-79-4]1,3-dimethyladamantane</strong> at 0 C. It is stirred over night at 0 C. and the mixture is poured at 0 C. onto 100 mL formamide in a round bottom flask which is provided with a drying tube. This mixture is stirred for 30 min at 0 C. and for 90 min at room temperature and 200 mL dichloromethane and 200 mL water are added. After phase separation, the organic phase is washed with water and 2 % NaHCO3-solution, is dried, over Na2SO4 and is freed from solvents at the rotary evaporator. The remaining oil is chromatographically purified (SiO2, CHCl3/acetone (20:1), Rf=0.39). 7.41 g (89.3%) of the formamide are obtained as a nearly colourless solid. 1H-NMR (CDCl3, TMS, 400.13 MHz): delta=0.87 ppm, s, 6 H; 1.15 ppm, s, 2 H; 1.2-1.35ppm, m, 4H; 1.35-1.55ppm, m, 4H; 1.65-1.78ppm, m, 2H; 2.10 -2.27 ppm, m, 1 H; 5.90 and 7.21 ppm; each br., s, 1 H; 8.02, 8.20 and 8.27 ppm, each s., 1 H. 13C-NMR (CDCl3, TMS, 100.61 MHz): delta=29,46, 30.01, 32.00, 40.21, 41.85, 47.63, 49.94, 51.83, 160.15/162.24 ppm. MS: m/z=207 (M+), 192, 150, 136, 106, 91, 79. |
|
|
Example 5Preparation of 1-formamido-3,5-dimethyladamantane[0067] <strong>[702-79-4]1,3-dimethyladamantane</strong> containing 0.05% or less of the impurity 1 ,3,5, trimethyladamantane, as prepared in Example 2, is treated with nitric acid followed by sulfuric acid at 0 0C. The reaction is stirred over night at 0 0C. The reaction mixture is poured onto 100 ml_ formamide (at 0 0C) in a round bottom flask which is equipped with a drying tube. The reaction is stirred at 0 0C for 30 min and then at room temperature for 90 min. Dichloromethane and water are then added. The organic phase is removed and washed with water and a 2 % NaHCO3-solution, dried over Na2SO4 and concentrated in vacuo. The resulting oil is purified via column chromatography to yield the title compound as a solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping