|
palladium; |
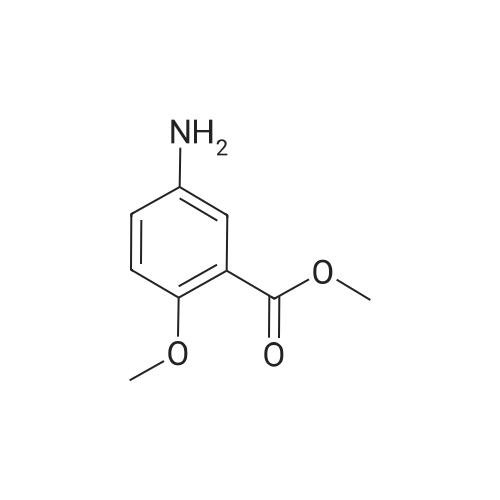
Combine methyl 2-methoxy-5-nitrobenzoate (13.3 g, 63 mmol) and methanol. Add 5% palladium-on-carbon (0.66 g). Hydrogenate on a pressure apparatus at 50 psi. After 17 hours, filter through celite to remove the catalyst and evaporate the filtrate in vacuo to give a residue. Combine the residue and dichloromethane and extract with water. Dry the organic layer over Na2SO4, filter, and evaporate in vacuo to give methyl 2-methoxy-5-aminobenzoate. Rf=0.18 (silica gel, ethyl acetate/methanol 1/1). Elemental Analysis calculated for C9H11NO3: C, 59.66; H, 6.12; N, 7.73. Found: C, 59.44; H, 6.04; N, 7.62. |
|
palladium; |
Combine methyl 2-methoxy-5-nitrobenzoate (13.3 g, 63 mmol) and methanol. Add 5% palladium-on-carbon (0.66 g). Hydrogenate on a pressure apparatus at 50 psi. After 17 hours, filter through celite to remove the catalyst and evaporate the filtrate in vacuo to give a residue. Combine the residue and dichloromethane and extract with water. Dry the organic layer over Na2SO4, filter, and evaporate in vacuo to give methyl 2-methoxy-5-aminobenzoate. Rf=0.18 (silica gel, ethyl acetate/methanol 1/1). Elemental Analysis calculated for C9H11NO3: C, 59.66; H, 6.12; N, 7.73. Found: C, 59.44; H, 6.04; N, 7.62. |
|
palladium; |
Combine methyl 2-methoxy-5-nitrobenzoate (13.3 g, 63 mmol) and methanol. Add 5% palladium-on-carbon (0.66 g). Hydrogenate on a pressure apparatus at 50 psi. After 17 hours, filter through celite to remove the catalyst and evaporate the filtrate in vacuo to give a residue. Combine the residue and dichloromethane and extract with water. Dry the organic layer over Na2SO4, filter, and evaporate in vacuo to give methyl 2-methoxy-5-aminobenzoate. Rf=0.18 (silica gel, ethyl acetate/methanol 1/1). Elemental Analysis calculated for C9H11NO3: C, 59.66; H, 6.12; N, 7.73. Found: C, 59.44; H, 6.04; N, 7.62. |
|
With hydrogen;5%-palladium/activated carbon; In ethyl acetate; under 1064.07 Torr; for 5h; |
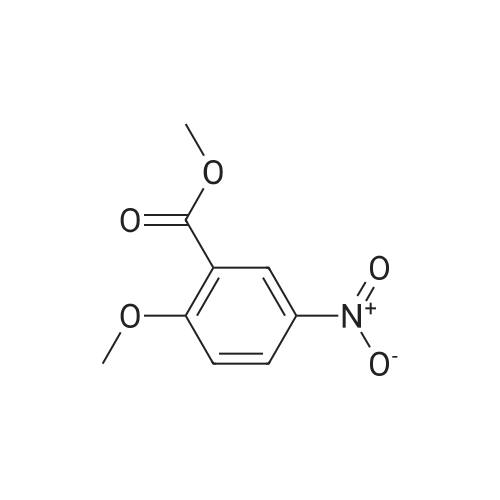
The 2-{2-methoxy-4-[6-methoxy-7-(N-methylcarbamoyl)quinolin- i5 4-yloxy]phenyl} acetic acid used as a starting material was prepared as follows :-A mixture of methyl 2-methoxy-5-nitrobenzoate (20.3 g), 5% platinum-on-carbon catalyst (1.5 g) and ethyl acetate (300 ml) was stirred under 1.4 atmospheres pressure of hydrogen for 5 hours. The catalyst was removed by filtration and the filtrate was evaporated. There was thus obtained methyl 5-amino-2-methoxybenzoate (17 g); 1H NMR: (CDCl3) 3.8420 (s, 3H), 3.89 (s, 3H), 6.86 (m, 2H), 7.19 (m, IH); Mass Spectrum: M+H+ 182. |
|
With hydrogen;platinum on carbon; In ethyl acetate; under 1064.07 Torr; for 5h; |
A mixture of methyl 2-methoxy-5-nitrobenzoate (20.3 g), 5% platinum-on-carbon catalyst (1.5 g) and ethyl acetate (300 ml) was stirred under 1.4 atmospheres pressure of hydrogen for 5 hours. The catalyst was removed by filtration and the filtrate was evaporated. There was thus obtained methyl 5-amino-2-methoxybenzoate (17 g); 1H NMR: (CDCl3) 3.84 (s, 3H), 3.89 (s, 3H), 6.86 (m, 2H), 7.19 (m, 1H); Mass Spectrum: M+H+ 182. |
|
With hydrogen;palladium(II) hydroxide/carbon; In methanol; under 760.051 Torr; for 18h; |
Methyl 2-methoxy-5-nitrobenzoate (6.21 g, 29.4 MMOL) was suspended in MEOH (100 mL) and 20% Pd (OH) 2/C (500 mg) was added. The mixture was stirred under a hydrogen atmosphere (1 atm) for 18 h. The catalyst was removed by filtration and the solvent evaporated under reduced pressure (5.256 g). |
| 15 g |
With palladium 10% on activated carbon; hydrogen; In methanol; under 3620.13 - 4137.29 Torr; |
A solution of methyl 2-methoxy-5-nitrobenzoate (20.0g, 0.097 mol) in methanol (300 mL) and 10% Pd/C (5.0 g) was stirred under hydrogen atmosphere under 70-80 psi pressure in Parr apparatus for 4-5 h. The reaction mass was filtered and the obtained filtrate was concentrated to afford 15.0 g of desired product. 1H NMR (300 MHz, DMSO d6): δ 3.67 (s, 3H), 3.74 (s, 3H), 4.96 (br s, 2H), 6.75 (dd, J = 2.4 Hz, 1H), 6.85 (d, = 8.7 Hz, 1H), 6.92 (s, 1H); MS (m/z): 182.25 (M+H)+. |
|
palladium; |
Combine methyl 2-methoxy-5-nitrobenzoate (13.3 g, 63 mmol) and methanol. Add 5% palladium-on-carbon (0.66 g). Hydrogenate on a pressure apparatus at 50 psi. After 17 hours, filter through celite to remove the catalyst and evaporate the filtrate in vacuo to give a residue. Combine the residue and dichloromethane and extract with water. Dry the organic layer over Na2SO4, filter, and evaporate in vacuo to give methyl 2-methoxy-5-aminobenzoate. Rf=0.18 (silica gel, ethyl acetate/methanol 1/1). Elemental Analysis calculated for C9H11NO3: C, 59.66; H, 6.12; N, 7.73. Found: C, 59.44; H, 6.04; N, 7.62. |
|
With hydrogen;5%-palladium/activated carbon; In methanol; under 2585.81 Torr; for 17h; |
Combine methyl 2-methoxy-5-nitrobenzoate (13.3 g, 63 mmol) and methanol. Add 5% palladium-on-carbon (0.66 g). Hydrogenate on a pressure apparatus at 50 psi. After 17 hours, filter through celite to remove the catalyst and evaporate the filtrate in vacuo to give a residue. Combine the residue and dichloromethane and extract with water. Dry the organic layer over Na2SO4, filter, and evaporate in vacuo to give methyl 2-methoxy-5-aminobenzoate. Rf=0.18 (silica gel, ethyl acetate/methanol 1/1.). Elemental Analysis calculated for C9H11NO3: C, 59.66; H, 6.12; N, 7.73. Found: C, 59.44; H, 6.04; N, 7.62. |
|
palladium; |
Combine methyl 2-methoxy-5-nitrobenzoate (13.3 g, 63 mmol) and methanol. Add 5% palladium-on-carbon (0.66 g). Hydrogenate on a pressure apparatus at 50 psi. After 17 hours, filter through celite to remove the catalyst and evaporate the filtrate invacuo to give a residue. Combine the residue and dichloromethane and extract with water. Dry the organic layer over Na2 SO4, filter, and evaporate invacuo to give methyl 2-methoxy-5-aminobenzoate. Rf =0.18 (silica gel, ethyl acetate/methanol 1/1). Elemental Analysis calculated for C9 H11 NO3: C, 59.66; H, 6.12; N, 7.73. Found: C, 59.44; H, 6.04; N, 7.62. |
|
palladium; |
Combine methyl 2-methoxy-5-nitrobenzoate (13.3 g, 63 mmol) and methanol. Add 5% palladium-on-carbon (0.66 g). Hydrogenate on a pressure apparatus at 50 psi. After 17 hours, filter through celite to remove the catalyst and evaporate the filtrate in vacuo to give a residue. Combine the residue and dichloromethane and extract with water. Dry the organic layer over Na2 SO4, filter, and evaporate in vacuo to give methyl 2-methoxy-5-aminobenzoate. Rf =0.18 (silica gel, ethyl acetate/methanol 1/1). Elemental Analysis calculated for C9 H11 NO3: C, 59.66; H, 6.12; N, 7.73. Found: C, 59.44; H, 6.04; N, 7.62. |
|
palladium; |
Combine methyl 2-methoxy-5-nitrobenzoate (13.3 g, 63 mrnol) and methanol. Add 5% palladium-on-carbon (0.66 g). Hydrogenate on a pressure apparatus at 50 psi. After 17 hours, filter through celite to remove the catalyst and evaporate the filtrate in vacuo to give a residue. Combine the residue and dichloromethane and extract with water. Dry the organic layer over Na2 SO4, filter, and evaporate in vacuo to give methyl 2-methoxy-5-aminobenzoate. Rf =0.18 (silica gel, ethyl acetate/methanol 1/1). Elemental Analysis calculated for C9 H11 NO3: C, 59.66; H, 6.12; N, 7.73. Found: C, 59.44; H, 6.04; N, 7.62. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping