|
|
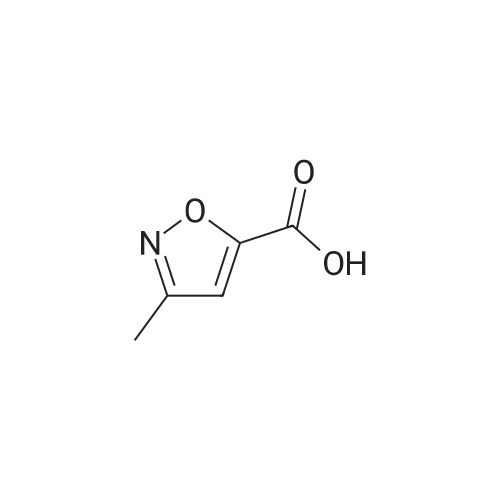
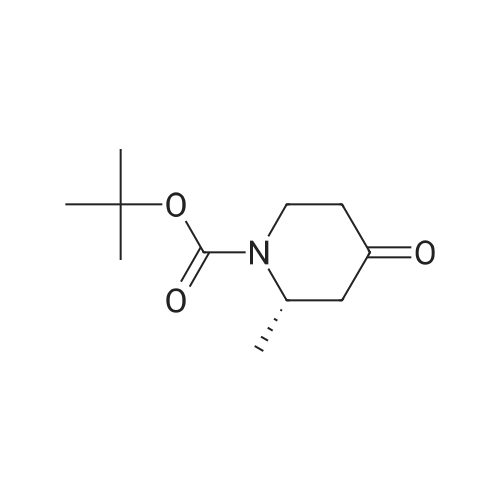
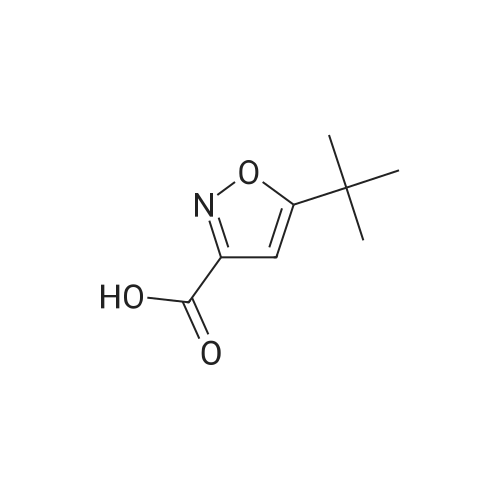
[00261] Isobutyl chloroformate (0.281 g, 2.06 mmcl) was added dropwise to a solution of 5-methylisooxazole-3-carboxylic acid (0.25 g, 1 .7 mmol) in THE (2.5 mL), followed by addition of N-methylmorpholine (0.208 g, 2.06 mmol). The reaction mixture was stirred at rt for 30 mm and then filtered to remove solids. In a separate flask, a solution of LHMDS (1 .3 M in THE, 2.6 mL, 3.4 mmol) was added dropwise to a solution of tert-butyl (S)-2-methyl-4-oxopiperidine-1- carboxylate (0.37 g, 1 .7 mmol) in THE (2.5 mL) at 0 00 under a nitrogen atmosphere. The reaction was stirred at 0 00 for 1 0 mm and then cooled to -78 00. To this reaction mixture, the filtrate containing the mixed anhydride from the first flask was added dropwise at -78 00. After the addition was complete, the reaction mixture was stirred at rt for 2 h. The reaction mixture was then acidified with aqueous 1 N HCI. The mixture was extracted with EtOAc (twice). The combined organic extracts were dried over Na2SO4, filtered and concentrated to give a mixture of crude tert-butyl (2S)-2-methyl-5-(5-methylisoxazole-3-carbonyl)-4-oxopiperidine- 1- carboxylate and tert-butyl (2S) -2-methyl-3-(5-methylisoxazole-3-carbonyl)-4-oxopiperidine- 1- carboxylate (0.43 g) which was used without further purification. MS m/z 323.5 (M+H).[00262] Hydroxylamine hydrochloride (0.56 g, 8.0 mmol) and N,N-diisopropylethylamine (2.1 g,16 mmol) were added to a solution of crude tert-butyl (25)-2-methyl-5-(5- methylisoxazole-3-carbonyl)-4-oxopiperidine- 1 -carboxylate and tert-butyl (25)-2-methyl-3-(5- methylisoxazole-3-carbonyl)-4-oxopiperidine-1-carboxylate (0.43 g) in ethanol (5 mL). The mixture was stirred at rt for 12 h and then concentrated. The residue was diluted with DOM and washed with water. The organic layer was dried over Na2504, filtered, and concentrated to give a mixture of crude tert-butyl (25)-4-(hydroxyimino)-2-methyl-5-(5-methylisoxazole-3- carbonyl)piperidine- 1 -carboxylate and tert-butyl (25)-4-(hydroxyimino)-2-methyl-3-(5- methylisoxazole-3-carbonyl)piperidine-1 -carboxylate (0.39 g) which was used without further purification.Step 3: (S)-6-methyl-3-(5-methyllsoxazol-3-yI)-4, 5,6, 7-tetrahydroisoxazolo[4,3-c]pyridine and (S)-4-methyl-3-(5-methylisoxazol-3-yI)-4, 5,6, 7-t etra hydrois oxazolo[4 , 3-c]pyridine [00263] A solution of HCI (4.0 M in dioxane, 1 .5 mL, 6.2 mmol) was added to a solution of crude tert-butyl (25)-4-(hydroxyim ino)-2-methyl-5-(5-methylisoxazole-3-carbonyl)piperidine- 1 -carboxylate and tert-butyl (25)-4-(hydroxyim ino)-2-methyl-3-(5-methylisoxazole-3- carbonyl)piperidine-1 -carboxylate (0.39 g) in DOM (4 mL). The mixture was stirred at 70 00 for1 h, then cooled to rt. The reaction was quenched with a solution of saturated aqueousNaHCO3 and then extracted with DCM (twice). The combined organic layers were dried overNa2SO4, filtered, and concentrated to give a mixture of crude (S)-6-methyl-3-(5-methylisoxazol-3-yl)-4,5,6,7-tetrahydroisoxazolo[4,3-c]pyridine and (S)-4-methyl-3-(5-methyl isoxazol-3-yl)-4,5,6,7-tetrahydroisoxazolo[4,3-c]pyridine (0.26 g) which was used without further purification.MS m/z 220.2 (M+H).[00264] Phenyl N-(3,4,5-trifluorophenyl)carbamate (see WO 2018011163 Al) (0.31 g, 1 .2 mmol) and N,N diisopropylethylamine (310 mg, 2.4 mmol) were added to a solution of crude (S)-6-methyl-3-(5-methylisoxazol-3-yl)-4,5,6,7-tetrahydroisoxazolo[4,3-c]pyridine and (5)- 4-methyl-3-(5-methylisoxazol-3-yl)-4,5,6,7-tetrahydroisoxazolo[4,3-c]pyridine (0.26 g) in acetonitrile (3 mL). The mixture was stirred at rt for 1 h and then quenched with water. The mixture was extracted with EtOAc (twice) and the combined extracts were dried over Na2504, filtered and concentrated. Silica gel column chromatography (EtOAc/heptane) provided a mixture of (S)-6-methyl-3-(5-methylisoxazol-3-yl)-N-(3,4,5-trifluorophenyl)-6,7- dihydroisoxazolo[4,3-c]pyridine-5(4H)-carboxamide and (S)-4-methyl-3-(5-methylisoxazol-3-yl)- N-(3,4,5-trifluorophenyl)-6,7-dihydroisoxazolo[4,3-c]pyridine-5(4H)-carboxamide (0.10 g). The mixture was re-purified by chiral SEC (Chiralpak AD-H column, 0.1% DEA in MeOH) to give:Example 25. (S)-6-methyl-3-(5-methylisoxazol-3-yl)-N-(3,4, 5-trifluorophenyl) -6,7- dthydroisoxazolo[4,3-c]pyridine-5(4H)-carboxamide[00265] (0.042 g, Fr-l). MS m/z 393.3 (M+H). 1H NMR (400 MHz, ODd3) 5 ppm 7.20 (dd, J = 9.5, 6.1 Hz, 2H), 6.66 (s, 1 H), 6.56 (s, 1 H), 5.23 (q, J = 6.6 Hz, 1 H), 4.95 (d, J = 15.9 Hz, 1 H), 4.56 (d, J = 15.9 Hz, 1 H), 3.12 (dd, J = 16.4, 5.7 Hz, 1 H), 2.96 (dd, J = 16.4, 1.6 Hz,H), 2.60 (s, 3H), 1.24 (d, J = 7.0 Hz, 3H).Example 26. (S)-4-methyl-3-(5-methylisoxazol-3-yl)-N-(3, 4, 5-trifluorophenyl) -6,7- dihydroisoxazolo[4, 3-c]pyridine-5(4H)-carboxamide[00266] (0.025 g, Fr-2). MS m/z 393.3 (M+H). 1H NMR (400 MHz, ODd3) 5 ppm 7.18 (dd, J = 9.5, 6.0 Hz, 2H), 6.65 (s, 1H), 6.54 (s, 1H), 5.46 (q, J = 6.7 Hz, 1H), 4.60 (dd, J = 14.1, 5.7 Hz, 1 H), 3.26 (td, J = 14.0, 13.4, 3.9 Hz, 1 H), 3.11 -3.01 (m, 1 H), 2.95 (ddd, J = 17.1, 12.6,... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping