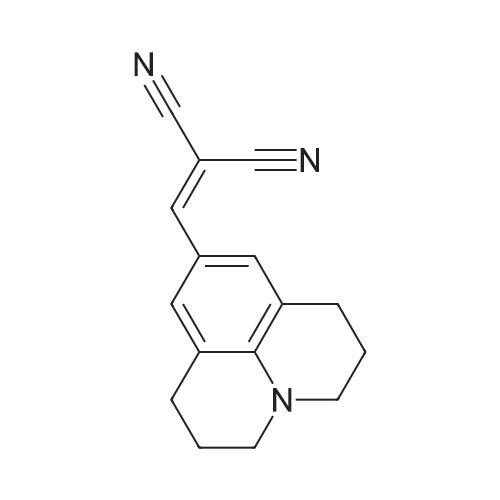
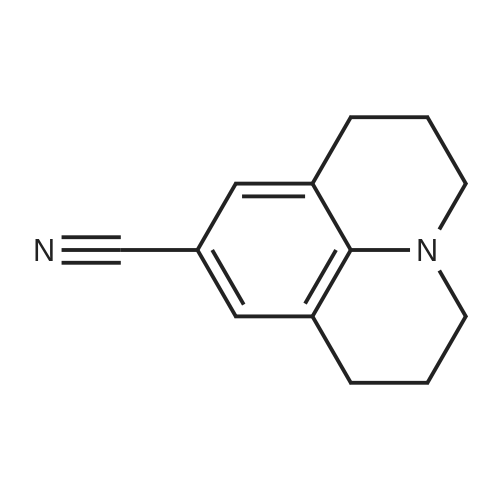
| 92% |
With trichlorophosphate; In dichloromethane; at 20℃; for 4h;Inert atmosphere; |
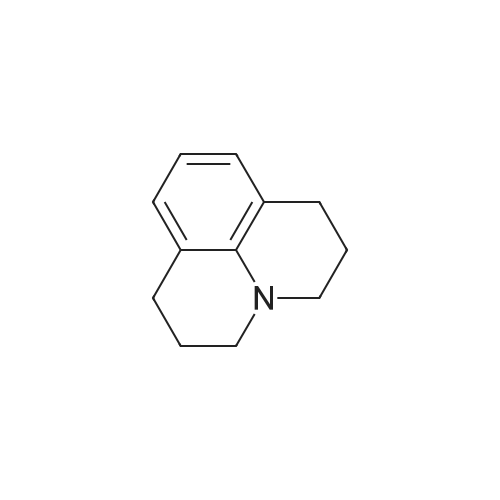
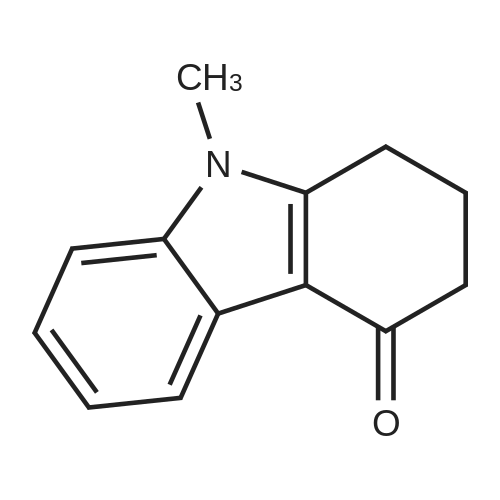
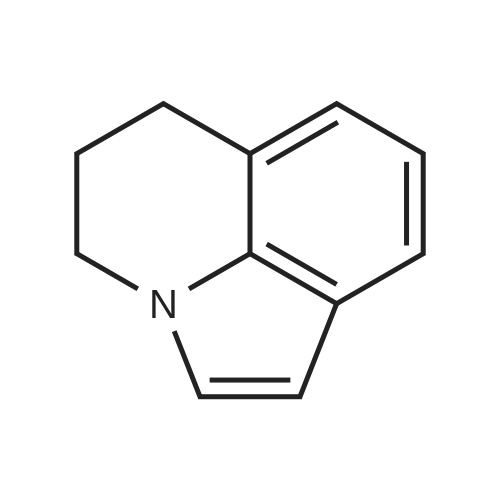
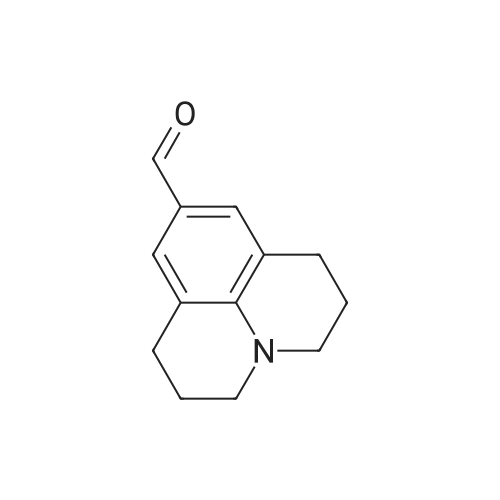
Julolidine (0.5 g, 2.89 mmol), DMF (0.255 g, 3.49 mmol)and POCl3 (0.535 g, 3.49 mmol) were dissolved in DCM(15 mL) and the mixture was stirred at room temperaturefor 4 h under an inert argon atmosphere. The solution?scolor turned green and the degree of advancement was followedby TLC. The solution was treated with aq. NaOH(2 M) and the crude product then was extracted withEt2O. After two aqueous washings, the organic phase wasdried on MgSO4, filtered and concentrated under vacuum.The product was then purified on column chromatographyusing 40%-50% Et2O: Hexane was used as the eluentto give 0.48 g (83.08%) (the reaction yield was upto 92% when 1.5 g julolidine was used as starting material)of a light yellow solid product.1H NMR (300 MHz,CDCl3: = 9597 (s, 1 H), 7.29 (s, 1 H), 3.308 (t, J =57 Hz, 4 H), 2.787 (t, J = 62, 4 H), 2.002-1.92 (m, J =63, 4 H). |
| 80% |
|
2,3,6,7-Tetrahydro-1H,5H-pyrido[3,2,1-ij]quinoline-9-carbaldehyde was synthesized according to the described method [Kauffman, Joel M.; Imbesi, Steven J.; Aziz, Mohammed Abdul - Organic Preparations and Procedures International, 2001, vol. 33, 6, p. 603 - 613] with some modifications: to a magnetically stirred solution of POCl3 (2.2 ml, 23 mmol) in DMF (2 ml) julolidine (2) (2 g, 11.6 mmol) and DMF (2 mL) under argon was added dropwise at 0 C. Then the mixture was allowed to stir for 3 h at rt (TLC). Ammonium hydroxide was added for neutralization and the solution was deluted with ethyl acetate and washed with water several times. The residue was purified by column chromatography (silica gel, 10% ethyl acetate/hexane). Yield 1.8 g (80%). 1H NMR (300 MHz, CDCl3): delta 9.63 (s, 1H), 7.28 (s, 2H), 3.31 (t, J=5.8 Hz, 4H), 2.81 (t, J= 6.2 Hz, 4H), 1.97-2.06 (m, 4H). |
| 71% |
With trichlorophosphate; In N,N-dimethyl-formamide; at 90℃; for 4.5h; |
The synthesis of 9-formyljulolidinewas carried outmodifying a reported procedure [45,46]. In brief,phosphorous oxychloride (1.1 mL, 11.55 mmol) was added dropwise to N,N-dimethyl-formamide(2 mL, 25.85 mmol) at 0 C. A solution of julolidine (2.0015 g, 11.55 mmol) in DMF (3.5 mL, 45.24 mmol)was then added and the mixture was stirred at 90 C for 4.5 h. The solution was allowed to coolat room temperature (rt) and neutralized to pH 6-8 by the addition of a saturated sodium acetatesolution (~30 mL). After stirring overnight at rt, a greenish-yellow solid precipitate was recoveredvia filtration, washed with water (30 mL) and dried under high vacuum. The crude product waspurified through column chromatography on silica gel using ethyl acetate/CHCl3 (70/30 v/v) aseluent mixture. 1.65 g of FJUL were recovered (71% yield). FT-IR (KBr, cm-1): 2758, 1651, 1594,1527, 1321. 1H-NMR (CDCl3): delta (ppm) = 9.6 (s, 1H, CHO), 7.3 (s, 2H, aromatic), 3.3 (t, J = 5.8 Hz,4H, NCH2), 2.7 (t, J = 6.3 Hz, 4H, NCH2CH2CH2), 1.9 (m, 4H, NCH2CH2). 13C-NMR (CDCl3): delta (ppm) = 190.1 (-CHO), 147.9 (-N-C(-C-)=C-), 129.5 (-C(=C)-CH=C(-C)-CH=), 124.0 (-CH-(CH=)C-CHO),120.33 (-CH2-C(=C-)-CH(=C)), 50.0 (-N(-CH2)-), 27.7 (-N(-CH2-CH2-CH2-)-), 21.3 (-N(-CH2-CH2-CH2-)-).EI-MS m/z (%): 201 (100, M+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping