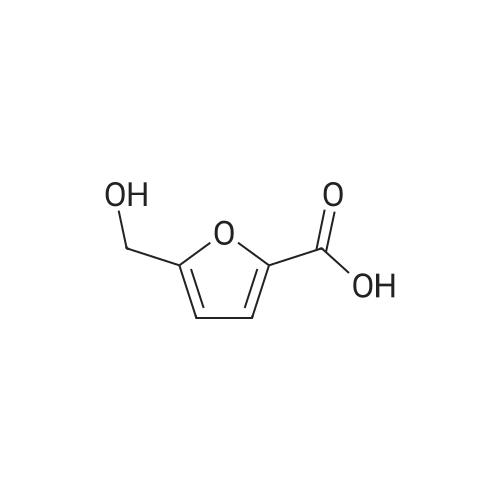
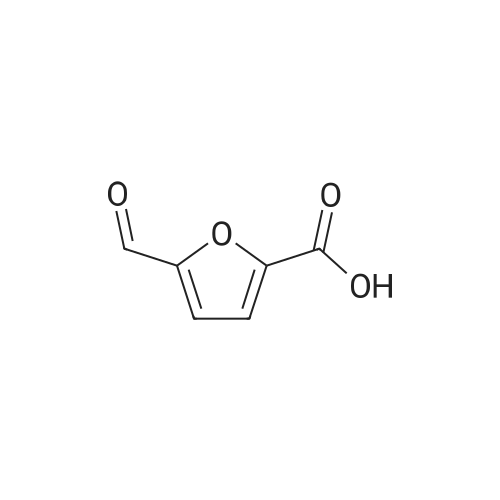
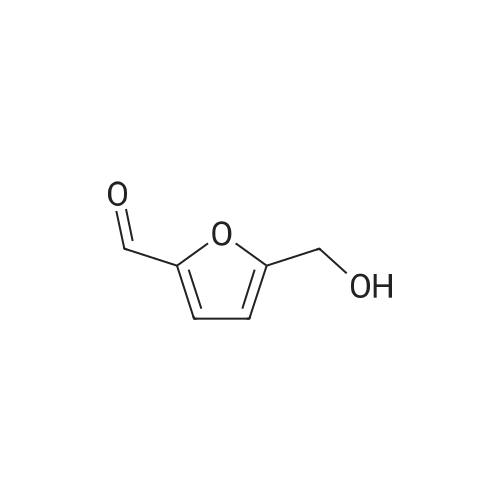
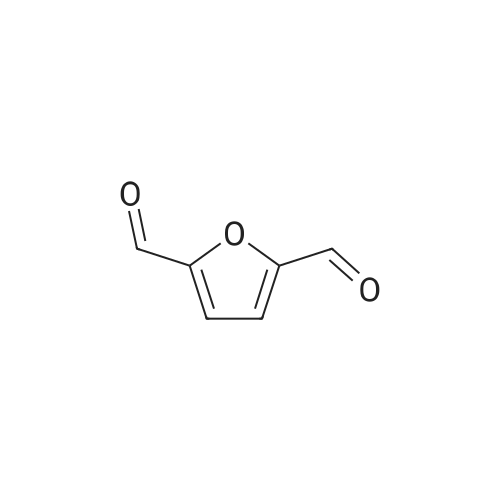
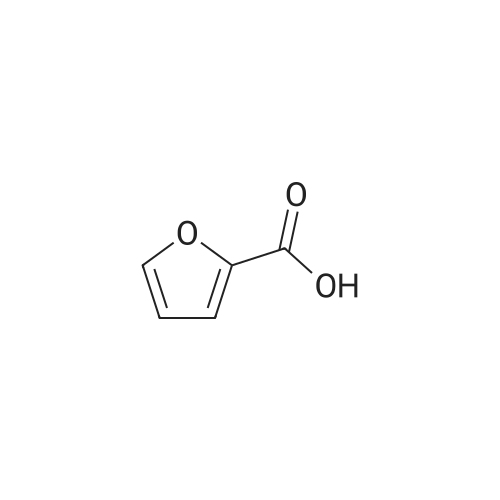
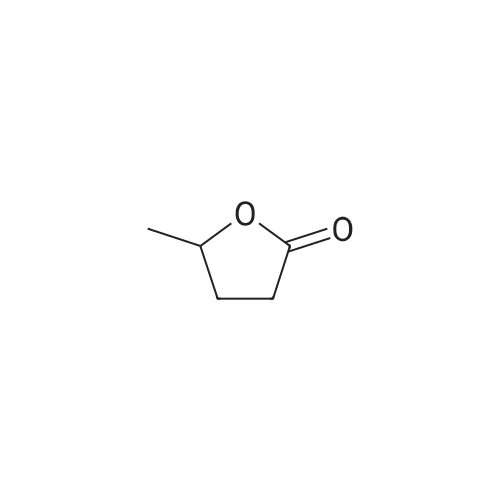
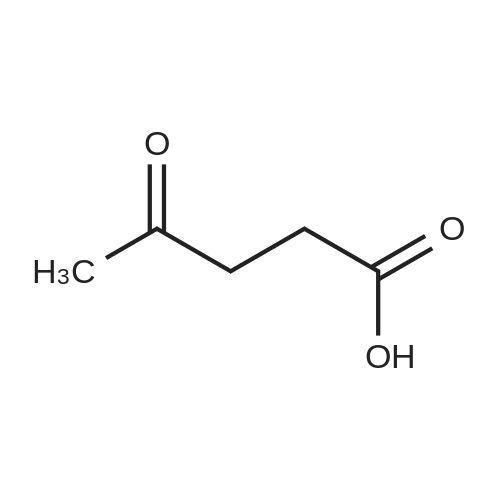
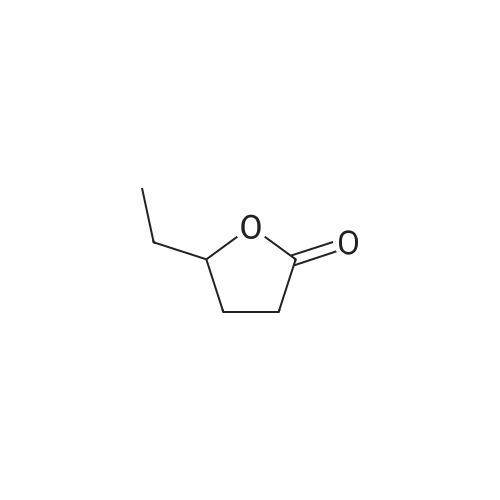
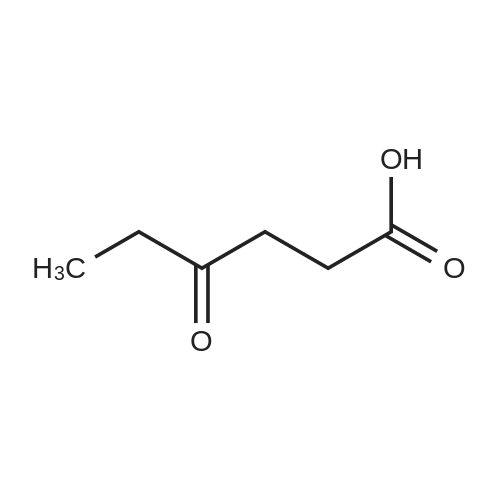
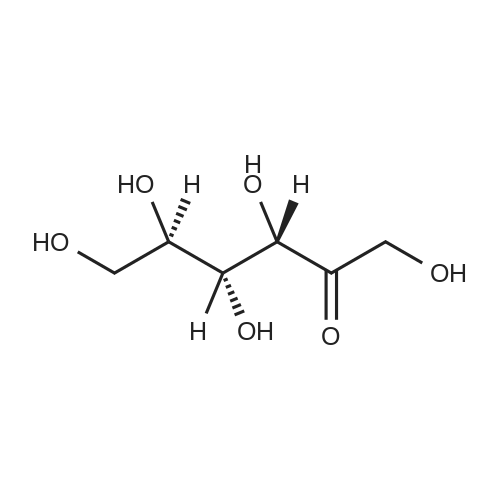
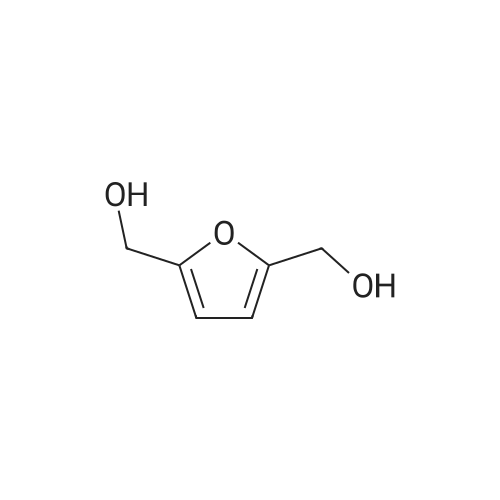
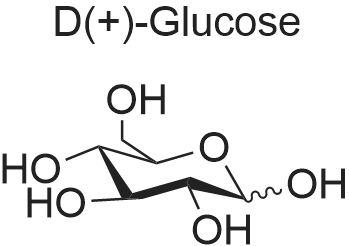
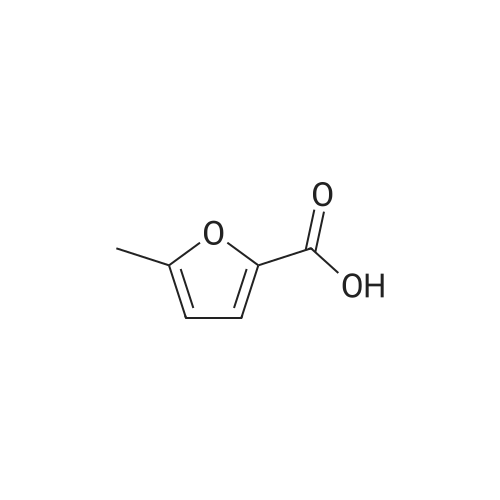
| 60%; 33% |
With bis-acetylacetonyl vanadium (II); In aq. phosphate buffer; at 37 - 80℃; for 16.0833h;pH 7; |
HMF (100 mM) was added to KPi buffer (500 mM pH 7.0). GOase M35 (103p1 of3.3mg/mL), PaoABC (ipI of 28.9mg/mL) and a metal complex (see Table 4) were added at 37 00 and the pH was continuously adjusted with NaHCO3 for a period of 16 hours. The reaction was heated to 80 00 for 5 minutes and left to cool. The solution containing denatured protein was centrifuged and the supernatant removed and analysed by RP20 HPLC. |
| 59.4%; 40.6% |
With oxygen; sodium hydroxide; In water; at 24.84℃; for 6h; |
The HMF oxidation reaction was carried out in a three-neck flask with an attached glass reflux condenser under oxygen flow (Figure 2). In each experiment, the reactor was filled with 1.0mmol of HMF and 5.0mmol of NaOH in 10mL of water. Then, 0.1 g of M/RGO (M = Pd, Rh, Ru, or Pt) was added to the reactor, and oxygen was introduced at a flow rate of 50mL min-1 with stirring under atmosphere pressure. After reaction, the catalyst was filtered off before the high-performance liquid chromatography (HPLC) measurement (AnimexHPX-87H column from Bio-Rad Laboratories Co., Ltd., 0.5mL min-1 flow rate, 10nM H2SO4 solvent, 323 K). The products were analyzed using a refractive index (RI) detector. |
| 39%; 45% |
With oxygen; sodium hydroxide; In water; at 60℃; under 2250.23 Torr; for 6h;Autoclave; Green chemistry; |
Take 0.317 grams of HMF, 2 grams of NaOH (20%), 3 ml of water into the reactor, the reaction vessel was equipped with a 10 ml polytetrafluoroethylene lined high pressure reactor, 0.30 g Au / H x Na 1-x Y (Au 2 wt%) as a catalyst, after the program is warmed to 60 C, filled with 0.3MPa oxygen, reaction for 6 hours, during the reaction, oxygen is continuously added, ensure that the reaction is carried out at constant temperature and pressure. The reaction product was centrifuged to the supernatant, analytical using HPLC. after testing, HMF conversion rate of raw materials is 100% HMFA yield of 39%, FDA yield of 45%, the reaction results in Table 1. |
|
With oxygen; sodium hydroxide; In water; at 60℃; under 7500.75 Torr; for 4h;Autoclave; |
The oxidation of 5-hydroxymethyl-2-furfural (HMF) was carriedout using an autoclave (Parr Instruments) reactor of 300 mLcapacity and equipped with a mechanical stirrer (0-1200 rpm) andprovision for measurement of temperature and pressure. The reactorwas charged with an aqueous solution (25 mL distilled water)containing the appropriate amount of 5-hydroxymethyl-2-furfural,base (NaOH) and catalyst (HMF/metal molar ratio = 100). The autoclavewas purged 3 times with O2 (5 bar) and then pressurized at10 bar. If not differently indicated, the temperature was increasedto 60 C and the reaction mixture was stirred at ca. 1000 rpm for4 h. At the end of the reaction, the reactor was cooled to room temperatureand the solution was filtered. Then, 4 mL of water wasadded to an aliquot of the reaction solution (1 mL) before analysiswith an Agilent Infinity 1200 liquid chromatograph equippedwith a Aminex HPX 87-H 300 mm×7.8 mm column using a0.005 M H2SO4 solution as the mobile phase. Identification of compoundswas achieved by calibration using reference commercialsamples. |
| 86.39%Chromat.; 9.2%Chromat. |
With disodium hydrogenphosphate; copper oxides/alumina; at 90℃; under 13501.4 Torr; for 0.166667h; |
i) Oxidation 1 - inNMP, Pt-C, 17 bar and 80C, Na2HPO4The process was carried out in a comparable, scaled up continouos lab-plant setup as used inthe examples above. Starting solution A was prepared by mixing 65.6g HMF and 400.OgNMP; solution B was a 15% solution of sodium phosphate, prepared by mixing 150.OgNa2HPO4 and 850.Og of deionized water.The flow rates used in this trial were 2.86m1/min solution A, 2.14m1/min solution B and 250.Onml/min for air. Further processing parameters were 10 minutes residence time at 90C and 18 bar. The catalyst used was copper oxide on aluminium oxide.The oxidation product mixture obtained from this trial contains, according to HPLC analysis, FDCA: 9.20%, HMFCA: 86.39%, FFCA: 0.13%, DFF: 0.36% and 2.82% of HMF. About 1% are unidentified side products.ii.) Extraction with ethyl acetateNMP extraction was carried out with ethylacetate. 50m1 reaction mixture was extracted six times with 40m1 ethyl acetate in each cycle.FDCA, HMFCA and FFCA remained completely in the water phase. Also a small amount of HMF was found in the water phase (0.5% according to HPLC). DFF completely went into the organic phase and was discarded. |
| 21%Chromat.; 74%Chromat. |
With oxygen; sodium hydrogencarbonate; In water; under 7500.75 Torr;Autoclave; Heating; |
HMF (0.2 mmol), NaHCO3 (0.4 mmol), and the catalyst (25 mg) were added to a 12 mL stainless steel autoclave containing 8 mL of deionized water. The autoclave was heated to 80 C and pressurized with O2 (10 bar) under vigorous stirring (900 rpm). During the reaction, 0.1 mL sample was taken at regular intervals of about 0.5-1 hours, filtered with 0.2 mum PTFE filters, diluted with water and analyzed using a high-performance liquid chromatograph (Shimadzu LC-20AD equipped a Bio-Rad Aminex HPX-87H column). Sulfuric acid (5 mM) at 333 Kwith a flow rate of 0.55 mL min-1 was used as an eluent. Each catalyst was tested at least twice to verify the reproducibility. The reproducibility of conversion levels and yields were within 5%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping




-1,3-Butanediol.png)