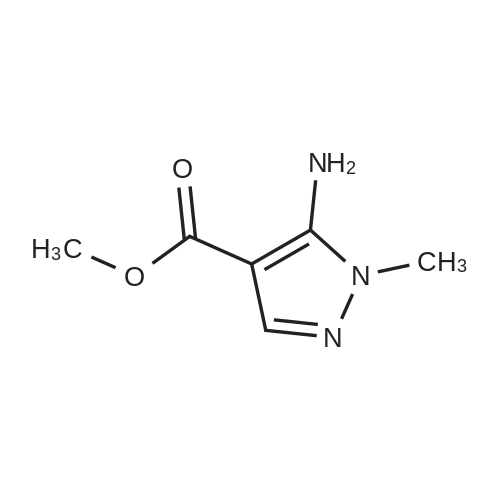
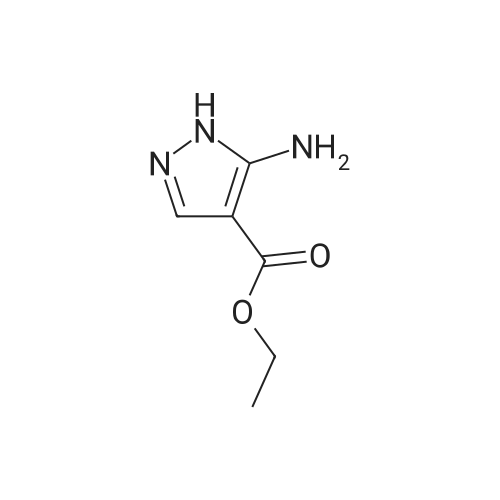
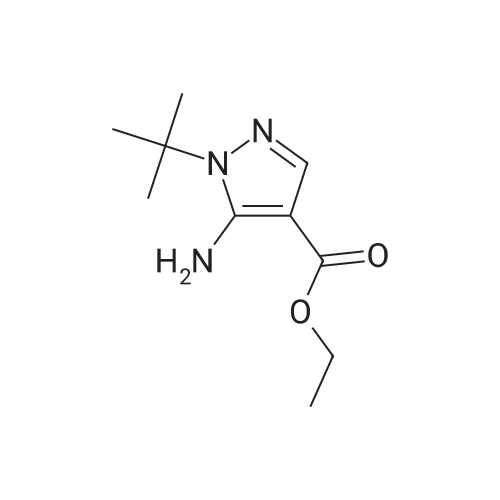
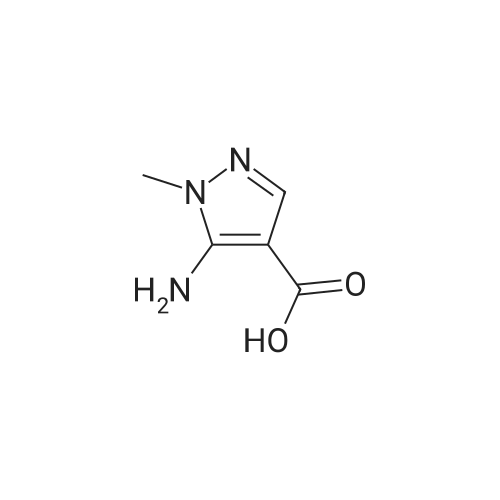
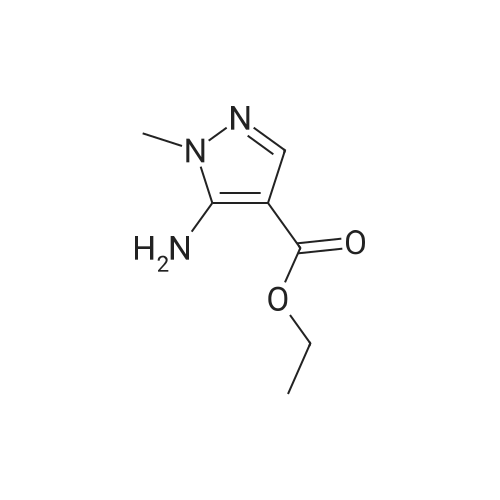
| 81% |
With tert.-butylnitrite; copper(ll) bromide; In acetonitrile; at 20 - 65℃; for 3.5h; |
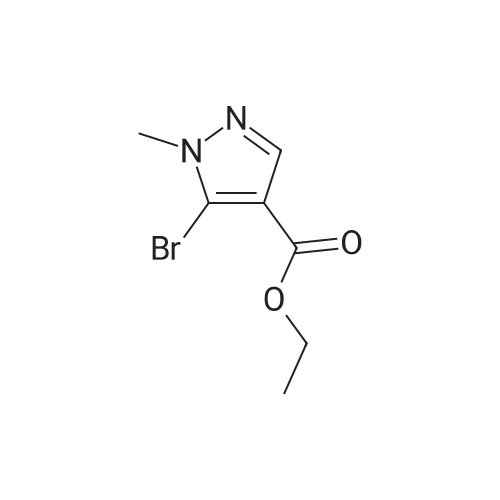
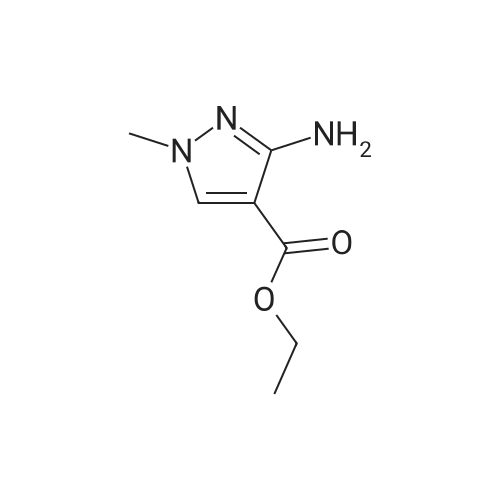
Intermediate 1: (5-Bromo-1-methyl-1H-pyrazol-4-yl)-(octahydro-quinolin-1-yl)methanone; Step 1. ; 5-Bromo-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester; To a mixture of t-butyl nitrite (29.5 mL, 221.5 mmol), cupric bromide (39.7 g, 177.5 mmol), and acetonitrile was added 5-amino-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester (25 g, 148 mmol) in portions over 30 minutes. The reaction mixture was stirred at ambient temperature for 2 h, then at 65 C. for 1 h. The mixture was then poured into 6N HCl (400 mL) and extracted with dichloromethane. After concentration in vacuo, the crude residue was purified by flash chromatography with a gradient of 0-20% ethyl acetate/hexanes to give 5-bromo-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester (28 g, 81%). |
| 68% |
With tert.-butylnitrite; copper(ll) bromide; In acetonitrile; at 60℃; for 2h;Inert atmosphere; |
5-Amino-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester (5.0 g, 29.6 mmol) was added portionwise to a mixture of tert-butyl nitrite (4.57 g, 44.3 mmol) and copper (II) bromide (7.92 g, 35.5 mmol) in acetonitrile (20 mL). The mixture was heated to 60C for 2 h. The resulting mixture was poured into 6M HCl (200 mL) and extracted with dichloromethane (3 x 250 mL). The combined organics was dried on magnesium sulfate and concentrated in vacuo. The crude material was purified by column chromatography (SiO2, 0% to 50% ethyl acetate in hexanes) to afford 5-bromo-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester as an off-white solid (4.7 g, 68%). 1H NMR (CDCl3) : 7.93 (s, 1H), 4.32 (q, J = 7.2 Hz, 2H), 3.92 (s, 3H), 1.36 (t, J = 7.0 Hz, 3H). |
| 66% |
With tert.-butylnitrite; copper(I) bromide; In acetonitrile; at 65℃; for 24h; |
Step 1. Synthesis of ethyl 5-bromo-1-methyl-1H-pyrazole-4-carboxylate Copper(II) bromide (99%, 20.0 g, 88.6 mmol) and tert-butyl nitrite (90%, 14.1 mL, 107 mmol) were combined in acetonitrile (65 mL) and heated to 65 C. Ethyl 5-amino-1-methyl-1H-pyrazole-4-carboxylate (10.0 g, 59.1 mmol) was slowly added portion-wise {Caution: gas evolution!} and the reaction was maintained at 65 C. for 24 hours. The mixture was cooled to room temperature, poured into aqueous hydrochloric acid (3 N, 600 mL), diluted with ethyl acetate (300 mL) and stirred for 10 minutes. The aqueous layer was extracted with ethyl acetate (150 mL), and the combined organic layers were dried over magnesium sulfate, filtered and concentrated in vacuo. The residue was purified via silica gel chromatography (Gradient: 5% to 100% ethyl acetate in heptane, with a 5-minute hold at 32%), affording the product as a pale yellow solid. Yield: 9.10 g, 39.0 mmol, 66%. LCMS m/z 233.3 (M+1). 1H NMR (500 MHz, CDCl3) delta 1.36 (t, J=7.1 Hz, 3H), 3.92 (s, 3H), 4.32 (q, J=7.1 Hz, 2H), 7.93 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping