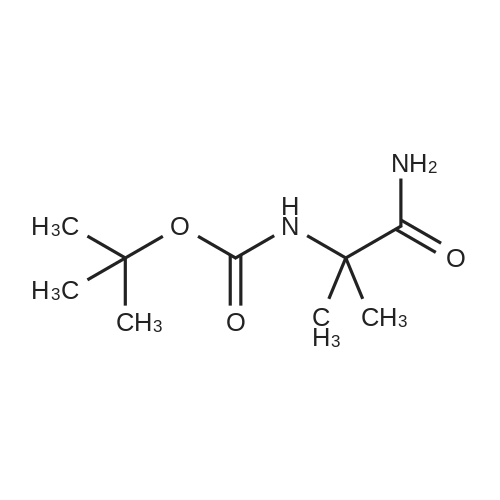
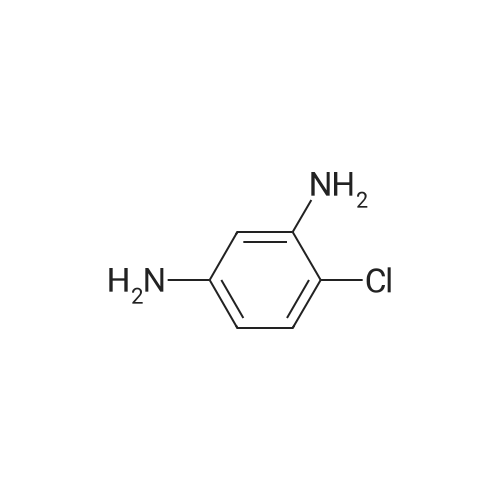
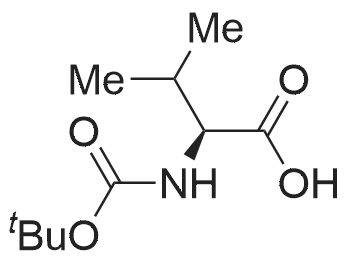
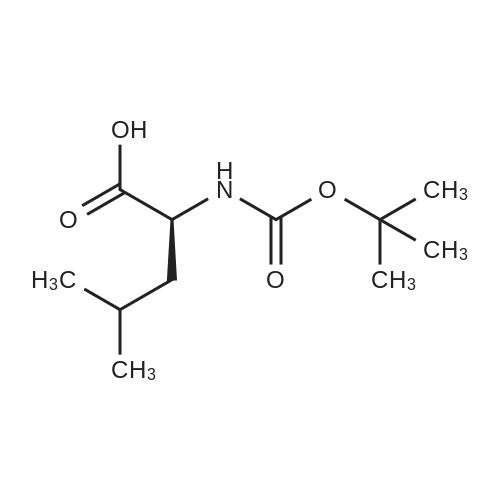
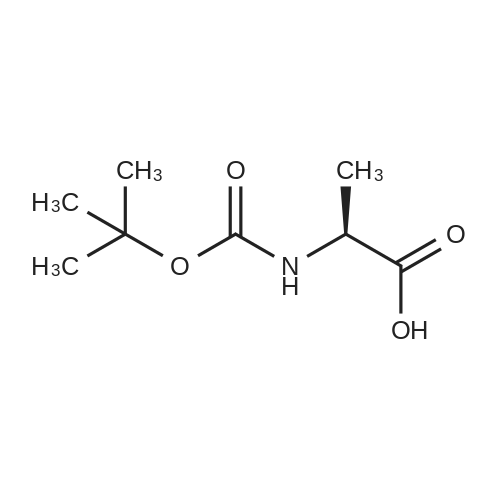
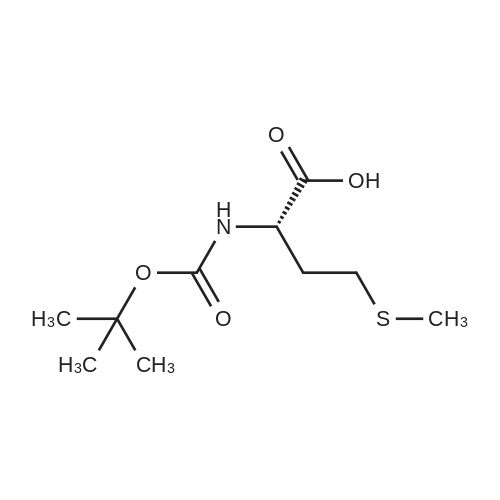
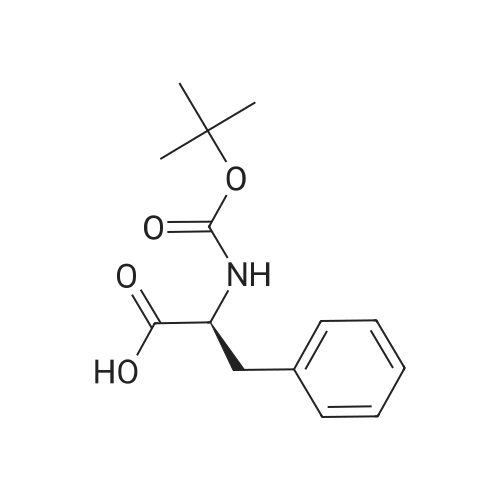
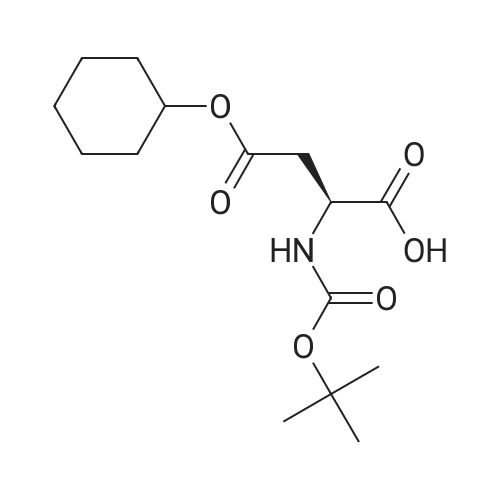
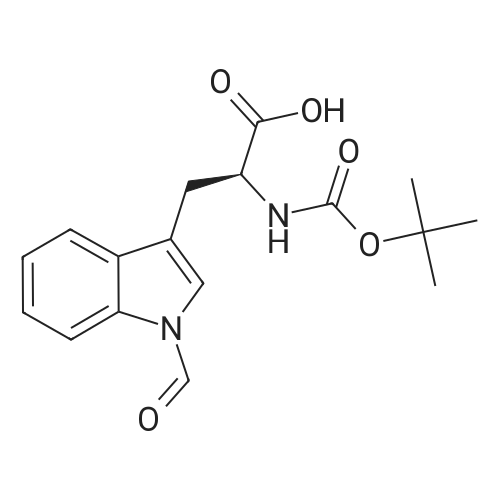
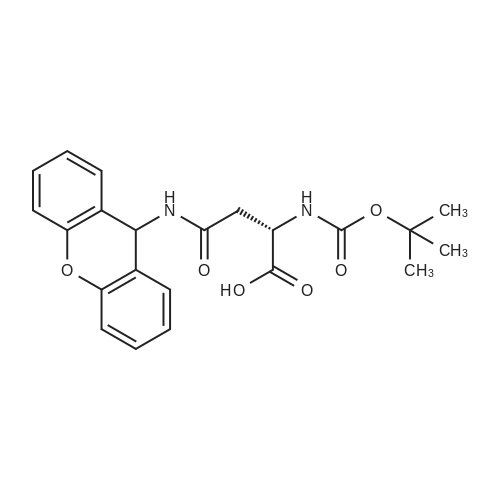
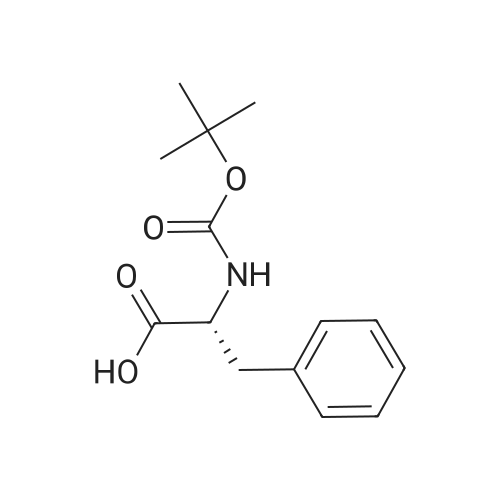
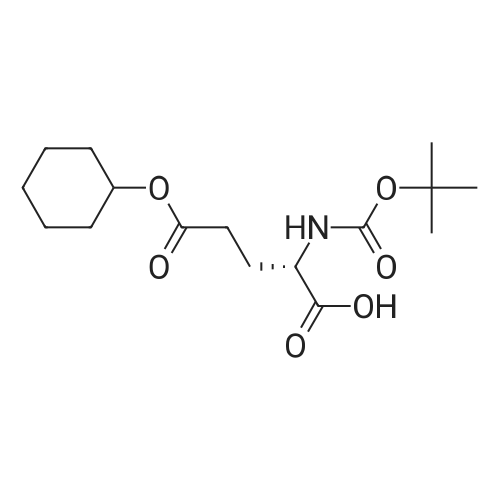
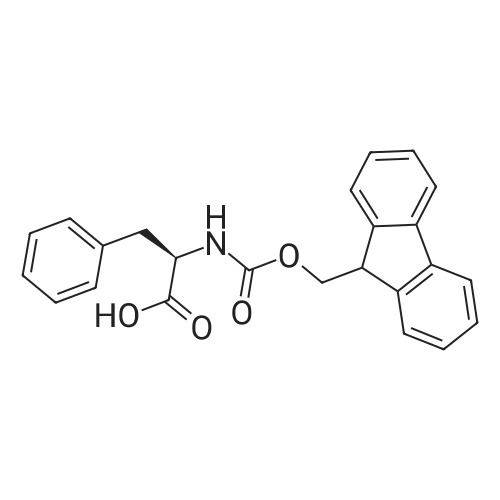
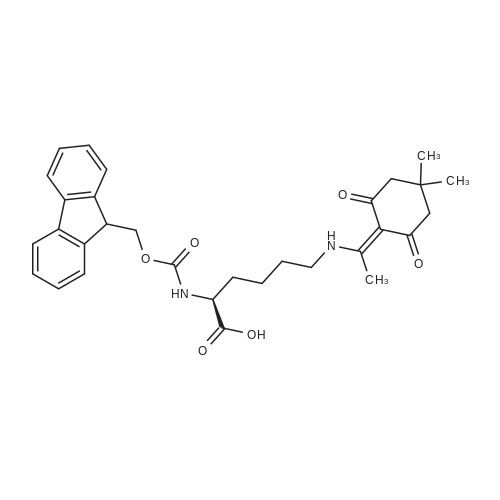
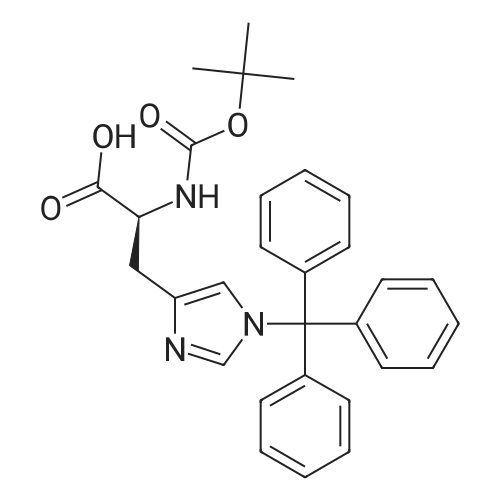
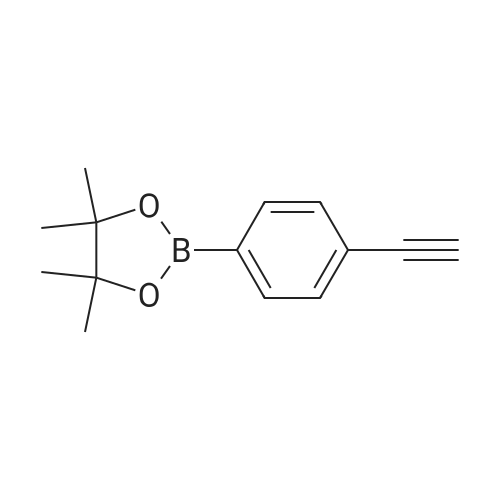
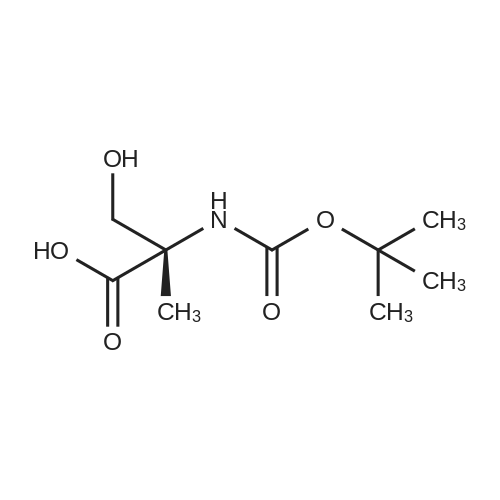
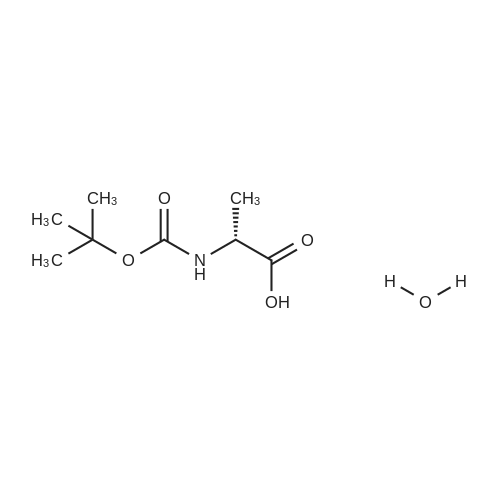
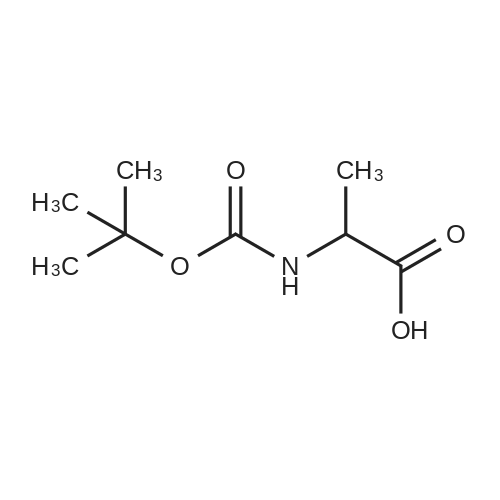
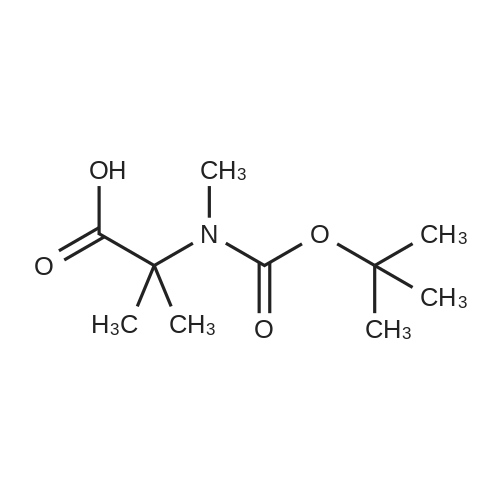
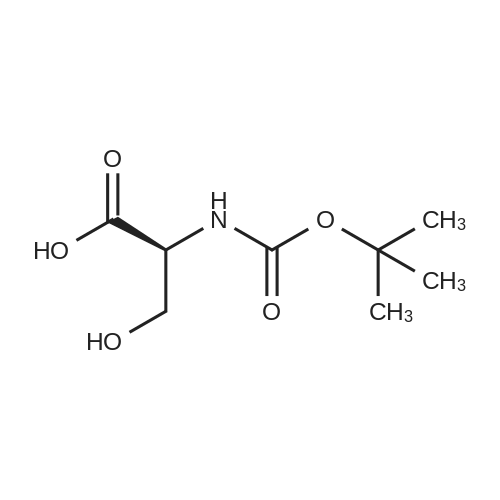
| 2 mg |
|
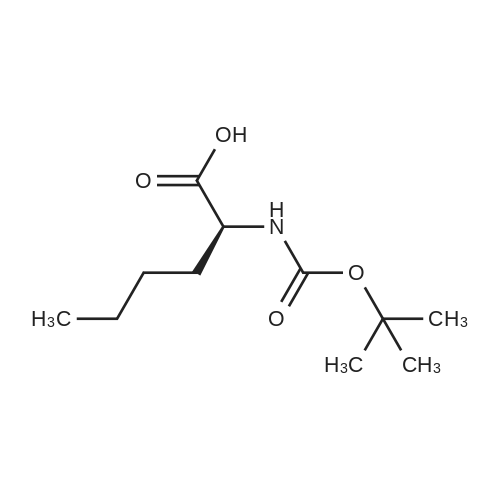
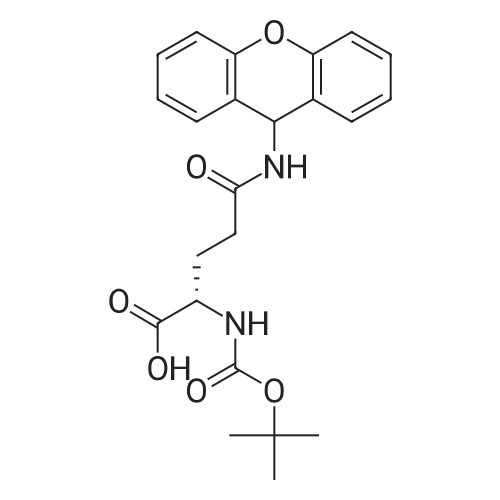
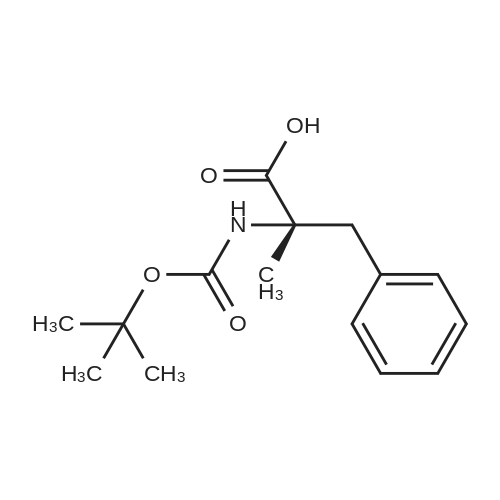
General procedure: The first four amino acids (from the C-terminus) of peptide 13 were coupled by automated peptide synthesis as described in the general section. Following coupling scheme was used: The remaining two amino acids of peptide 13 were coupled manually using a 5-fold molar excess of amino acid, FIOBt and DIC (75 pmol each) in DMF as solvent. In addition, the whole sequence of peptide 15 was prepared by manual synthesis on a Rink amide AM resin using the same reagent conditions. Following coupling scheme was applied: In case of peptide 15, the resin was treated with a capping solution of 10 % DIPEA and 10 % acetic anhydride in DCM (15 min, 500 pi) after loading with the first C-terminal amino acid. Removal of Fmoc protecting groups after each manual coupling step was accomplished by using 20 % piperidine in DMF (2 x 10 min, 500 mI each). Peptides 14 and 16 were entirely prepared by automated peptide synthesis as described in the general section. The coupling scheme was as follows: The first two C-terminal amino acids of peptide 17 and 18 were coupled by automated peptide synthesis as described in the general section. Following coupling scheme was used:The remaining three amino acids of peptide 17 and 18 were coupled manually using a 3-fold or 5- fold molar excess of the amino acid and a 5-fold molar excess of FIOBt and DIC (75 pmol each) in DMF as solvent. The coupling scheme was as follows: Removal of Fmoc protecting groups after each manual coupling step was accomplished by using 20 % piperidine in DMF (2 x 10 min, 500 pi each). For the removal of the Mmt protecting group in peptide 13, the resin was treated with a cleavage mixture consisting of 2 % TFA, 5 % TIS in DCM (8 x 2 min, 1 ml each). After each deprotection step, the resin was washed with DCM. Finally, the resin was incubated with 5 % DIPEA in DCM (2 x 10 min, 1 ml each). For the cleavage of the Dde protecting group in all other peptides (14-18), the resin was treated with 2 % hydrazine in DMF (10 x 10 min, 1 ml each). In case of peptide 14, the building block Fmoc-L-Dap(Mtt)-OFI was coupled manually in 3-fold molar excess with a 5-fold molar excess of FIOBt and DIC (75 pmol each) to the e-amino group of the C- terminal lysine. DMF was used as solvent and the coupling time was approximately 16 h. For peptide 16, the building block Fmoc-L-Dap(Fmoc)-OFI was coupled manually in 5-fold molar excess with FIOBt and DIC (75 pmol each) in DMF to the C-terminal lysine side-chain. The coupling time was 4 h. Subsequently, removal of the Fmoc protecting groups was achieved by using 20 % piperidine in DMF (2 x 10 min, 500 mI each). 6-TAMRA was coupled manually to peptide 16 using a 2-fold molar excess of the fluorophore, FIATU and DIPEA (30 pmol each) in DMF as solvent for 5 h. Afterwards, removal of the Mtt protecting group in peptide 16 was performed as described for the Mmt deprotection above.The carbaboranes were coupled manually in 3-fold molar excess per free lysine or Dap amino group, except for mlJ9b, which was coupled in 1.5-fold molar excess per free amino group. Coupling reactions were prepared as follows: Peptides 13, 14 and 17: 3 eq. m9b, 5 eq. FIOBt and 5 eq. DIC in DMF as solvent. Peptide 15: 3 eq. bm9x, 4 eq. FIOBt and 4 eq. DIC in DMF. Peptide 16: 3 eq. mlJ9b, 4 eq. FIOBt and 4 eq. DIC in DMF. Peptide 18: 1.5 eq. mlJ9b, 2 eq. FIOBt and 2 eq. DIC in DMF. All coupling reactions were performed overnight for approximately 16 h. Cleavage of conjugates 13-15 and 17 from the resin and simultaneous side chain deprotection was accomplished using a mixture of TFA/TA/EDT (90:7:3, 1 ml) for 3 h. Cleavage of conjugates 16 and 18 from the resin was achieved using a mixture of TFA/FhO (95:5, 1 ml) for 3 h. The crude conjugates were precipitated and washed with an ice-cold mixture of hexane/diethyl ether (3:1, v/v), dissolved in ACN/FI2O and subsequently lyophilized. The first purification of the crude conjugate 13 was performed by preparative RP-HPLC using a C18- column (Phenomenex Jupiter 5u 300 A: 250 mm c 21.2 mm, 5 pm, 300 A) with a flow rate of 10 ml/min and a linear gradient of 50 % to 80 % eluent B in A over 30 min. Conjugate 13 had to be purified a second time using a XBridge C18-column (Waters XBridge Peptide BEH C18 OBD: 250 mm c 19 mm, 10 pm, 130 A) with a flow rate of 15 ml/min and a linear gradient of 50 % to 80 % eluent B in A over 30 min. For purification of conjugate 14, a XBridge C18-column (Waters XBridge Peptide BEH C18 OBD: 250 mm c 19 mm, 10 pm, 130 A) with a flow rate of 15 ml/min and a linear gradient of 30 % to 60 % eluent B in A over 30 min was applied. Purification of the conjugates 15-17 was achieved using a Kinetex C18-column (Phenomenex Kinetex 5u XB-C18: 250 mm c 21.2 mm, 5 pm, 100 A) with a flow rate of 15 ml/min. For conjugate 15 and 16, a linear gradient of 40 % to 70 % eluent B in A over 30 min was used, whereas for conjugate 17 a gradient of 50 % to 80 % eluent B in A over 30 min was applied. Purification of the... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping