| 91% |
With hydrogen;palladium 10% on activated carbon; In methanol; at 20℃; for 3h; |
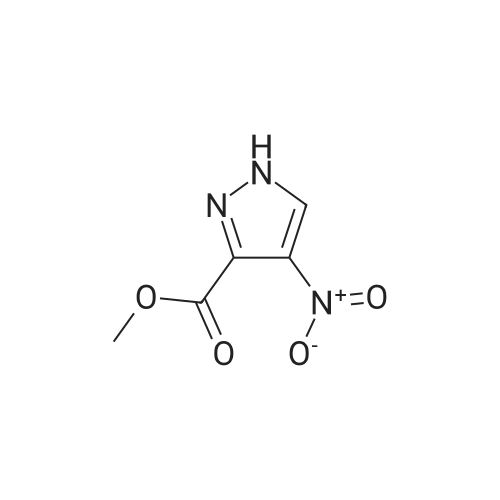
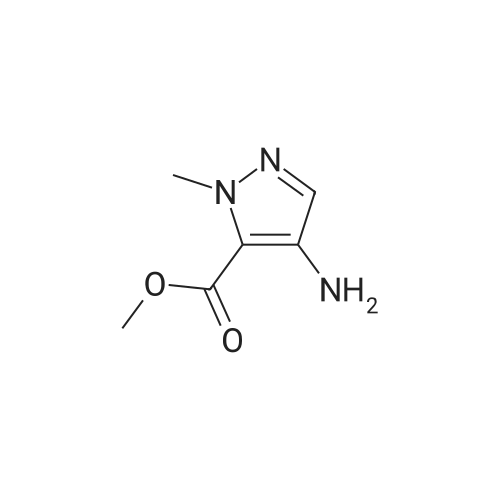
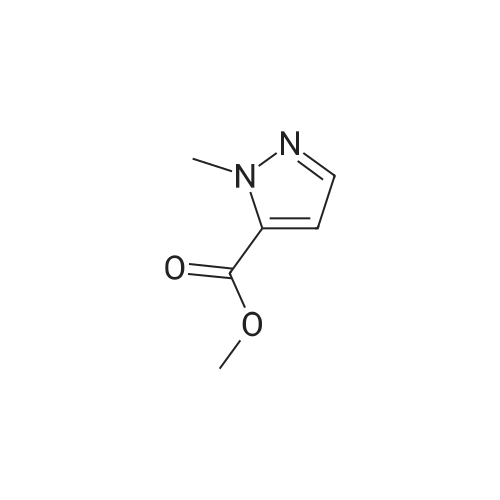
Compound 7: 10% wt. Pd/C (0.15 g, 0.14 mmol) was added to a solution containing 6 (0.26 g, 1.4 mmol) in 10 mL of methanol. The mixture was stirred under a hydrogen atmosphere at ambient temperature. After 3 hours, the reaction mixture was filtered thru a plug of Celite. The resulting filtrate was concentrated under reduced pressure to afford 7 (0.20 g, 91%), ES (+) MS m/e=156 (M+1). |
| 90% |
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; under 1551.49 Torr; for 5h; |
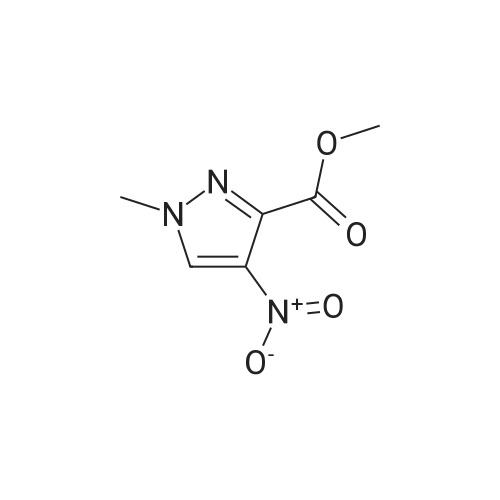
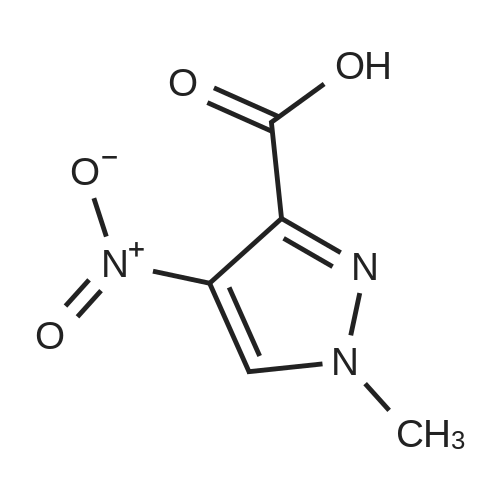
Methyl 2-methyl-4-nitro-2H-pyrazole-3-carboxylate (4.00 g, 21.6 mmol) was dissolved in methanol (120 mL), dry palladium on carbon (10% palladium, 1% water, 400 mg) was added. The reaction solution was allowed to react under 30 psi hydrogen pressure for 5 hours at room temperature. The reaction solution was filtered, and the filtrate was concentrated under reduced pressure to give methyl 4-amino-2-methyl-2H-pyrazole-3-carboxylate (3.00 g, as an off-white solid) with a yield of 90%. 1H NMR: (400 MHz, CDCl3) δ 7.10(s, 1H), 4.09(s, 2H), 4.02(s, 3H), 3.90(s, 3H). MS-ESI calcd. [M + H]+ 156, found 156. |
| 41% |
With hydrogen;palladium 10% on activated carbon; In methanol; under 30402.0 Torr; for 2h; |
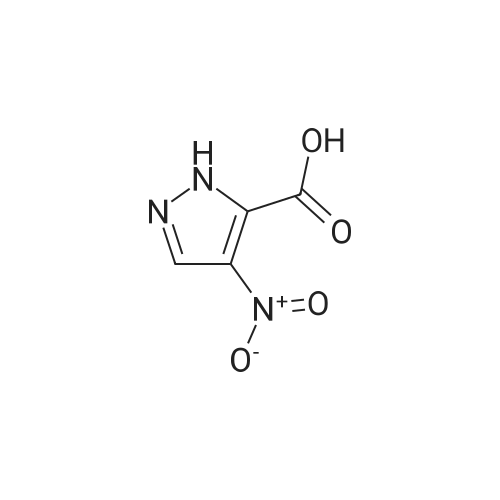
127 mg (0.69 mM) of the compound obtained in Preparative Example 13 was dissolved in 3 ml of methanol, to which 10% palladium/charcoal was added, and the resulting mixture was stirred under hydrogen pressure of 40 atm for 2 hours. After the reaction was terminated, the resulting reaction solution was filtered through cellite, and distilled under reduced pressure, to obtain 43 mg (41%) of the title compound.1H NMR (300 MHz, CDCl3) δ 3.92 (s, 3H), 4.04 (s, 3H), 4.09 (br, NH2), 7.08 (s, 1H).Mass: 155 (M+) |
| 41% |
With hydrogen;palladium 10% on activated carbon; In methanol; under 30402.0 Torr; for 2h; |
127 mg (0.69 mM) of the compound obtained in Preparative Example 13 was dissolved in 3 ml of methanol, to which 10% palladium/charcoal was added, and the resulting mixture was stirred under hydrogen pressure of 40 arm for 2 hours. After the reaction was terminated, the resulting reaction solution was filtered through cellite, and distilled under reduced pressure, to obtain 43 mg (41%) of the title compound.1H NMR(300MHz, CDCl3) δ 3.92(s, 3H), 4.04(s, 3H), 4.09(br, NH2), 7.08(s, IH). Mass : 155(M+) |
|
With hydrogen;palladium 10% on activated carbon; In tetrahydrofuran; at 20℃; for 6h; |
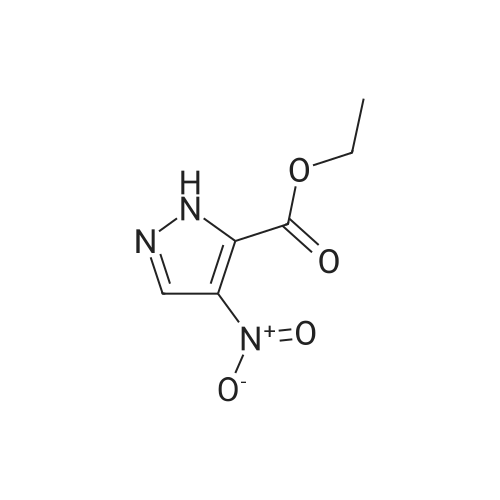
[0228] To a solution of methyl l-methyl-4-nitro-lH-pyrazole-5-carboxylate (1.5 g) in THF (20 mL) was added Pd/C (500 mg, 10%). The solution was stirred under a hydrogen atmosphere for 6 h at RT. The reaction mixture was filtered, dried over sodium sulfate, filtered, and concentrated to give 1.1 g of crude methyl 4-amino-l- methyl-lH-pyrazole-5-carboxylate. LCMS: 156 (M+H) +. |
| 4.71 g |
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; under 2068.65 Torr; for 4h; |
B) Methyl 4-amino-1-methyl-1H-pyrazole-5-carboxylate To a solution of methyl 1-methyl-4-nitro-1H-pyrazole-5-carboxylate (8.62 g) in methanol (100 mL), palladium-carbon (10%) (0.86 g) was added, and the mixture was stirred at room temperature for 4 hours in a hydrogen atmosphere (40 psi). Palladium-carbon was filtered off, and then, the filtrate was concentrated to obtain the title compound (4.71 g). 1H NMR (400 MHz, CDCl3) δ 3.91 (3H, s), 4.03 (3H, s), 4.15 (2H, brs), 7.07 (1H, s). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping