|
With dihydrogen peroxide;pH 7.2;aqueous phosphate buffer; UV-irradiation;Product distribution / selectivity; |
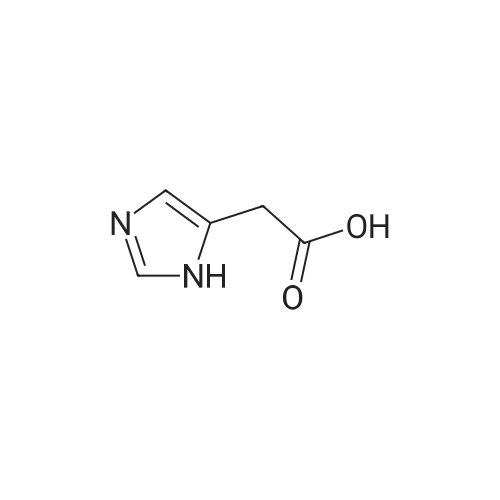
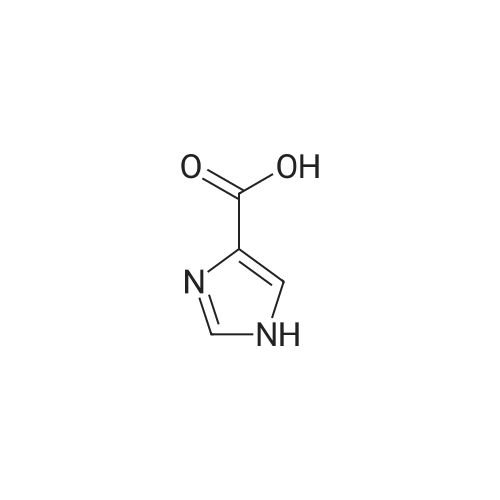
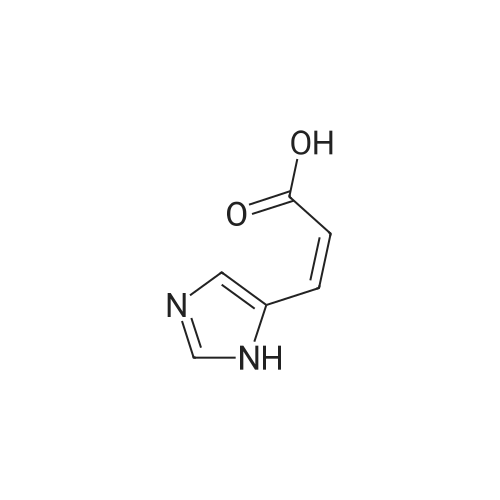
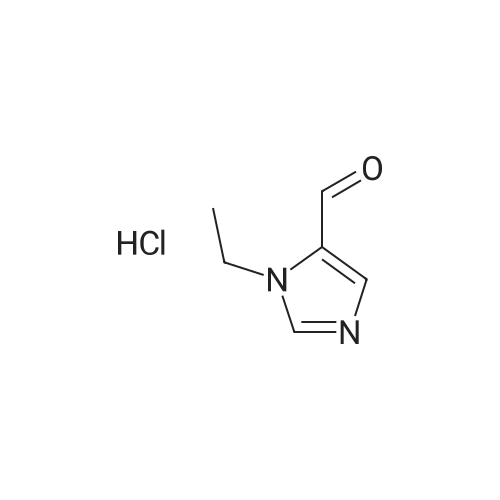
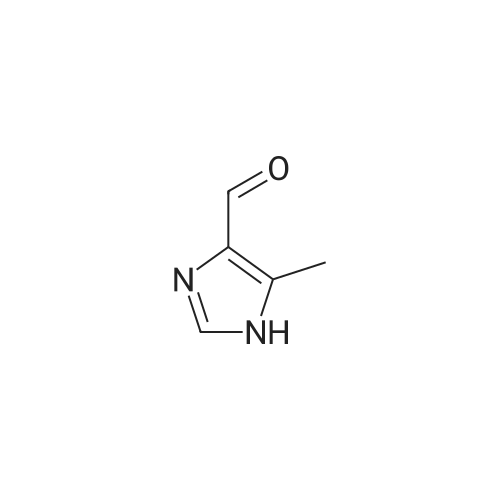
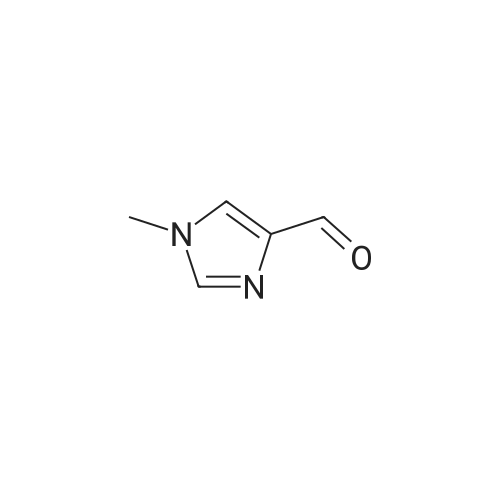
Photooxidation; A 1-cm quartz cuvette, filled with 1.4 mL sample, was placed in the parallel beam of a filtered 1000 W xenon arc lamp (Oriel, Stratford, CT). The samples were magnetically stirred during irradiation. To minimize infrared (heat) and visible radiation, the beam was passed through a water filter (7 cm), reflected by a dichroic mirror and filtered through a 1-mm UG11 filter. Short-wave cut off was achieved by passing the beam through WG280, WG305 or WG335 filters with 3 mm thickness each (Schott-Jena, Mainz, Germany). Xenon lamp emission filtered through WG280 included W-C, UV-B and W-A; through WG305 W-B and W-A and through WG335 only W-A was included. Two narrow bands in the W-B and W-A spectral regions were selected to monitor the xenon-arc emission. The probe of a calibrated EGG 550 radiometer (Salem, MA, USA) was equipped with a neutral density filter and narrow band filter type UV-M-IL (Schott-Jena) with a transmission maximum of 21 percent at 303 nm and a half-width of 11.5 nm to monitor UV-B or with a type UV-PIL (Schott-Jena) with a transmission maximum of 46 percent at 363 nm and a half-width of 7.7 nm to monitor UV-A. Transmission spectra of the optical filters were checked on a Perkin Elmer Lambda 40 UV/VIS spectrometer (Norwalk, CT, USA). Additional irradiations were performed with fluorescent tubes TL12, used as a UV-B source, and TL10R, used as a UV-A source (Philips, Eindhoven, The Netherlands), on samples that were magnetically stirred in small Petri dishes. The UV-B output was measured with an IL 443 phototherapy radiometer, fitted with a SEE 1240 silicon detector probe and the UV-A output with an IL 442A phototherapy radiometer with a SEE 115 detector probe (International Light, Newburyport, MA, USA); UCA photo-oxidation on a preparative scale Concentrations of trans-UCA and hydrogen peroxide were largely increased, as was the UV exposure, to obtain larger amounts of UCA photo-oxidation products as collected fractions from the reversed phase column for further analysis. A typical chromatogram is shown in Fig. 4. Four fractions, designated as Rt 8, Rt 10, Rt 14, Rt 17, were finally selected for identification (peak A, 1-3 in Fig.4). Prior to analysis, tetrabutylammonium was removed by solid phase extraction on C18 silica.Identification Rt 8 was identified as imidazole-4-carboxaldehyde (ImCHO). Its UV-spectrum was identical to the synthesized (see below) reference compound with an absorption maximum of 257 nm. Co-injection of Rt 8 with synthesized imidazole-4-carboxaldehyde resulted in a single chromatographic peak with a retention time of 8.13 minutes. Further evidence is to be collected (peak A in Fig.4). The amount of ImCHO in the photooxidized UCA sample was gradually reduced upon storage at -20°C. Rt 10 was identified as imidazole-4-acetic acid. Its UV-spectrum was identical with an absorption maximum of 213 nm. Mass spectrum was obtained with electrospray technique and the dry sample was treated with methanol/HCl and n-butanol/HCl before analysis. A peak at mass 140 was obtained after methylation and at mass 183 after butylation. Consequently, the mass of the original compound was 126. Co-injection of Rt 10 with commercially available imidazole-4-acetic acid resulted in a single chromatographic peak with a retention time of 8.98 minutes (peak 1 in Fig.4). Rt 14 was identified as imidazole-4-carboxylic acid (ImCOOH). Its UV-spectrum was identical to the commercially obtained reference compound with an absorption maximum of 226 nm. Proton resonance (1H-NMR) analysis was done in D2O, showing imidazolic protons in a ratio 1:1 with shifts of 7.76 and 7.53 ppm. Mass spectrum was obtained with electrospray technique and the dry sample was treated with methanol/HCl and n-butanol/HCl before analysis. A peak at mass 126 was obtained after methylation and at mass 169 after butylation. Consequently, the mass of the original compound was 112. Co-injection of Rt 14 with commercially available ImCOOH resulted in a single chromatographic peak with a retention time of 14.73 minutes (peak 2 in Fig.4). The amount of ImCOOH in the photooxidized UCA sample was gradually increased upon storage at -20° C. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping