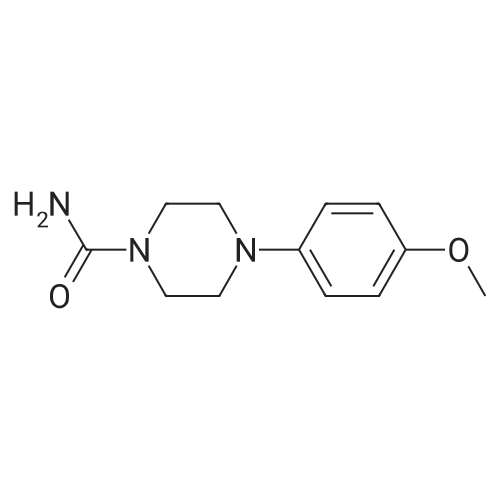
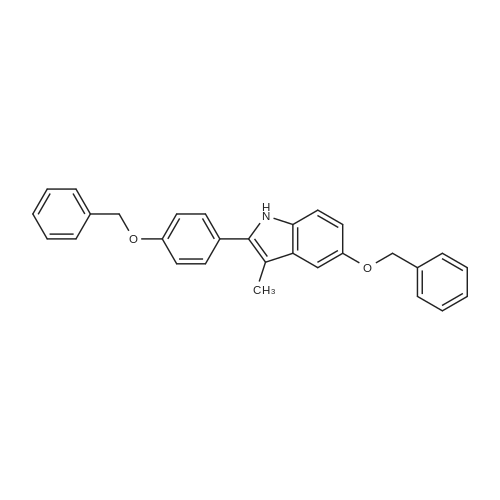
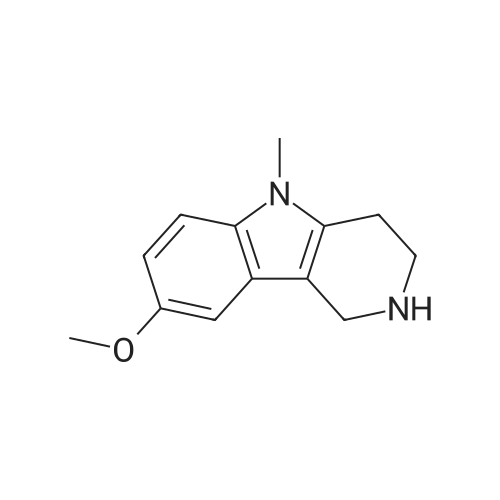
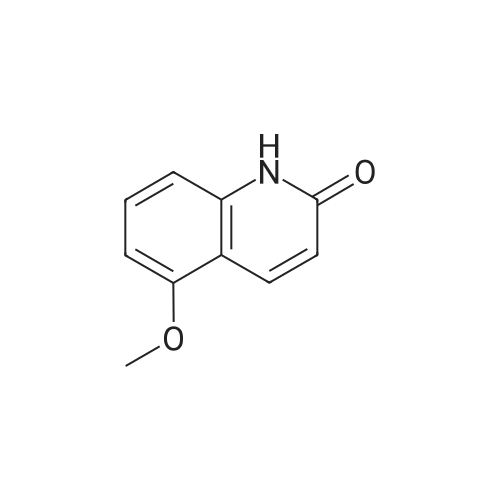
| 50% |
|
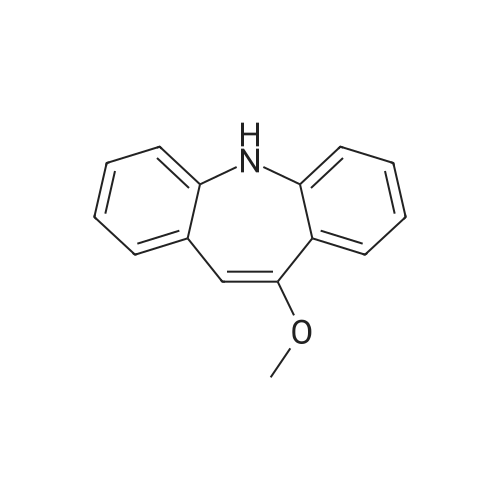
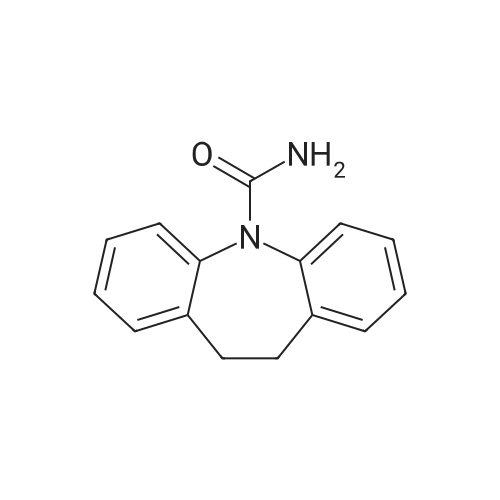
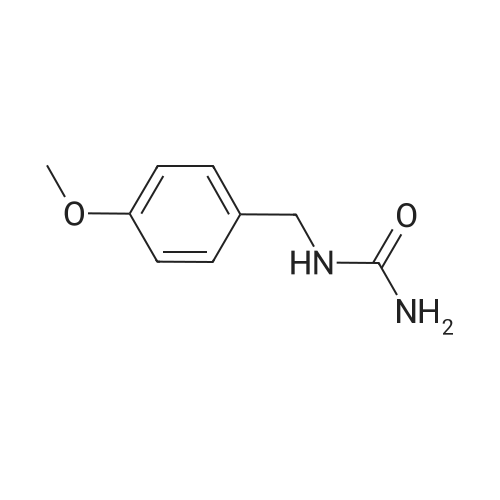
[10-METHOXY-5H-DIBENZ (B, 0AZEPINE] (29.9 g, 0.134 mol) was added to 300 ml acetonitrile in a three-neck flask (500 ml) equipped with a mechanical stirring apparatus. The mixture was stirred at room temperature for 15 minutes, and then [NAOCN] (18 g, 0.277 mol) was added, followed by pyridiniump-toluenesulfonate (75 g, 0.3 mol). The mixture was stirred at room temperature for about 4 hours, at which time TLC indicated that the reaction was complete (disappearance of [10-METHOXY-5H-DIBENZ (BAZEPINE).] 30 ml water was added, and the mixture stirred for 15 minutes. The solids were filtered and the filtrate was concentrated under vacuum. The semi-solid residue was triturated with acetone to provide a creamy solid, which was dried at [80] C to provide 18 g (0.068 mol, 50% yield) of [10-METHOXY-5H-DIBENZ [B2FLAZEPINE-5-CARBOXAMIDE (10-METHOXYCARBAMAZEPINE).] |
|
|
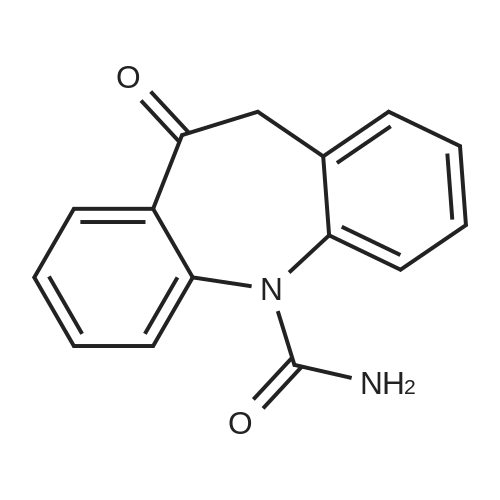
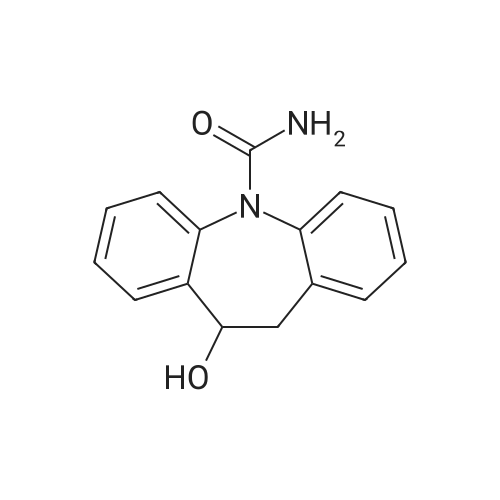
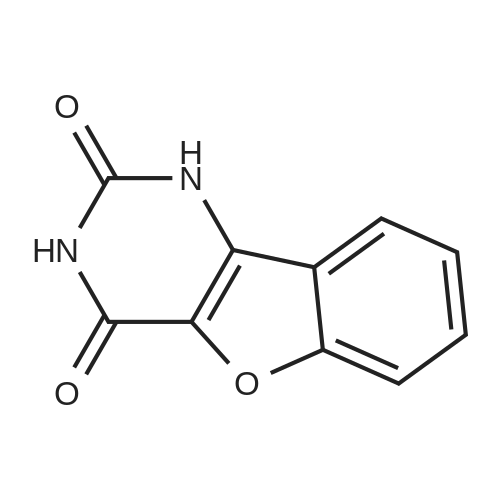
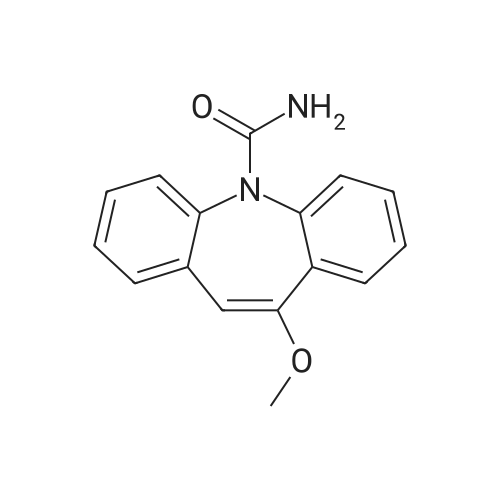
In a three-neck flask (500 ml), equipped with a mechanical stirring apparatus, 15 ml water was introduced, followed by pyridinium bromide (71.75 g, 0.45 mole; Chemadaa' (Nir Yitshak, Israel) ). The mixture was stirred at room temperature [(22 C)] for about 10 minutes. Then 250 ml toluene was added, followed by 50 g (0.224 mol) of [10-METHOXY-5H-] dibenz [[BZF] AZEPINE] (compound of formula (4), wherein R4 is methoxy). [Note: 10-methoxy-5H- dibenz [[B, T] AZEPINE] may be obtained from [IMINOSTILBENE] according to the process disclosed in U. S. Patent No. 5,808, 058. [10-METHOXY-5H-DIBENZ [B FLAZEPINE] also is commercially available from various suppliers, including Zhejiang Jiuzhou Pharmaceutical Co. , Ltd. (Zhejiang, China), and Ningbo Chongyangtang Biologic Tech Co. , Ltd. (Ningbo, China)] [NAOCN] (45 g, 0.69 mol ; OCI Corp. , South Korea) was added and the reaction was mixed for about 7-8 hours at room temperature [(22C).] After [7-8] hours, [125ML] of water was added, and the mixture was stirred for about 15 minutes. The resulting solid carbamate of formula (1), 10-methoxy-5H- dibenz [[B2TAZEPINE-5-CARBOXAMIDE,] was filtered and washed with 50 ml of water. The organic layer was separated and washed with water (2 x [50ML).] In a 500 ml three necked flask, the organic phase from above was introduced, to which the solid carbamate of formula (1) [(10-METHOXY-5H-DIBENZ [B2FLAZEPINE-5-CARBOXAMIDE)] was added to form a slurry. The mixture was heated to 89 C to clarify the solution, and 250 ml of 10% [HC1] was added dropwise with stirring. When thin layer chromatography indicated that the intermediate carbamate of formula (1) had been substantially consumed, the reaction mixture was cooled to room temperature and the mixture was stirred at this temperature for 15-30 minutes. The product, [10-OXO-10,] [11-DIHYDRO-5H-DIBENZ [B2FLAZEPINE-5-CARBOXAMIDE] (oxcarbazepine), was filtered and the crude oxcarbazepine cake was thoroughly washed with water until the pH reached 6-7. The mixture was then washed with toluene (50 ml), and the solids were dried to yield 34.4 g (0.137 mol) crude oxcarbazepine as a yellow-brown powder (yield relative to (2) is [61%).] The crude oxcarbazepine was slurried in 408 ml of boiling 80: 20 isopropanol : water for about 1 hour. The solid was separated by filtration and dried to afford 30.6 g oxcarbazepine [(89%] purification yield). Further purification was carried out in 765 ml of boiling 80: 20 isopropanol: water, hot filtration and cooling to room temperature. Filtration and drying of the solid precipitate afforded 26.5 g of purified oxcarbazepine (yield relative to starting material (2) is 47%, and purification yield is 87%).; The carbamoylation reaction was performed as in Example 1, except that [150] ml toluene was used instead of 250 ml. The resulting solid carbamate of formula (1), 10-methoxy-5H- dibenz [[B2FLAZEPINE-5-CARBOXAMIDE,] was isolated as described in Example 1. In a three necked flask, 330 ml of isopropyl alcohol, 78 ml of 12% HCl, and the solid 10- [METHOXY-SH-DIBENZ [B2FLAZEPINE-S-CARBOXAMIDE] were introduced. The mixture was heated to [78] [- 82 C] for about 1 hour. When TLC indicated that the carbamate of formula (1) was consumed, the reaction mixture was cooled to room temperature and stirred for 1 hour. The separated solids were filtered and washed with water to provide crude 10-oxo-10, [11-DIHYDRO-SH-DIBENZ [BFLAZEPINE-S-] carboxamide (oxcarbazepine), which was purified as described in Example 1. Yield relative to starting material of formula (2) was 43-45%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping