| 90% |
|
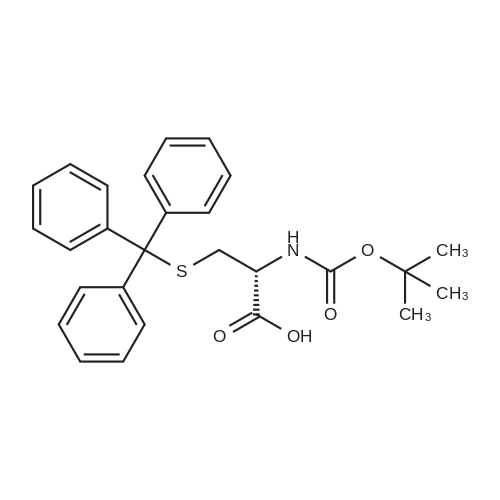
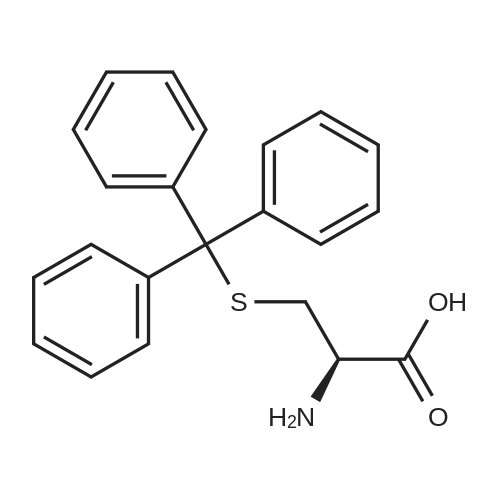
To a solution of 2[36] and [37] (5 g, 12.7 mmol) in NaOH 2N (80 mL) was added Boc2O (4.5 g, 20 mmol) and the reaction mixture was stirred at room temperature for 24 h. The aqueous solution was then acidified with HCl concentrated until pH 2 and extracted with CH2Cl2 (2 .x. 100 mL); the combined organic phases were washed with brine, dried and concentrated under reduce pressure to give without any further purification compound 3 (5.3 g, 90percent) as white foam.1H NMR (DMSO): delta (ppm) 1.37 (s, 9H, 3 .x. CH3); 2.29-2.58 (m, 2H, CH2); 3.76-3.79 (m, 1H, CH); 6.84 (d, J = 7.8 Hz, 1H, NH); 7.14-7.38 (m, 15H, trityl-H).MALDI-TOF MS: m/z 464.8 Da [M + H], C27H29NO4S, Mol. Wt.: 463.59. |
| 86.21% |
With sodium hydroxide; In 1,4-dioxane; water; at 20℃; for 8h;Cooling with ice; |
The compound <strong>[2799-07-7]S-trityl-L-cysteine</strong> (5 g, 13.76 mmol)Was dissolved in dioxane (40 ml)Water (20 ml),1M sodium hydroxide solution (14 ml)Stir under ice bath,Boc-anhydride (3.5 ml, 15.14 mmol) was added,The reaction naturally rose to room temperature,Stirring for 8 hours,TLC detection reaction is completed,The reaction mixture was concentrated to 20-25 ml,Ethyl acetate was added,A solution of sodium bisulfate was added dropwise under stirring in an ice bath,After adjusting the pH to 2-3,Extracted with ethyl acetate,The organic layer was washed with saturated brine,Then dried over sodium sulfate,concentrate,Column chromatography (methanol: dichloromethane 1percent -5percent),The product was a white solid (5.5 g, yield: 86.21percent). |
| 60% |
|
To the solution of Trt-Cys-OH (22.68 g, 62.5 mmol) in dioxane (60 mL) and water (125 mL) was added di-tert-butyldicarbonate (41 g, 187 mmol) at 45 0C, and the solution was adjusted with NaOH(4M) until pH = 9.5, and then stirred at the same temperature overnight. Once the reaction was done, water and dioxane was removed under reduced pressure. The residue was dissolved into water (150 mL) , extracted with ethyl acetate (2 x 100 mL) . The aqueous layer was adjusted to pH = 2 with dilute HCl while in an ice bath, and then the aqueous layer was extracted with ethyl acetate. The combined ethyl acetate layers were washed with water, dried over magnesium sulfate. Removal of the solvent under vacuum yielded a yellow oil. The residue was then dissolved into ethyl ether and carefully added a 1 : 1 mixture of ethyl ether and hexane while stirring to <n="32"/>precipitate out the white solid in 60percent yield. Ic: 1H NMR (300 MHz, CDC13): deltal.46 (s, 9H), 2.69 (br, 2H), 4.21 (s, IH), 4.97 (s, IH), 7.20-7.44 (m, 15H), 10.2 (br, IH); 13C NMR (75.5 MHz, CDC13): delta 28.1, 33.5, 52.4, 144.1, 155.4, 175.1 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping