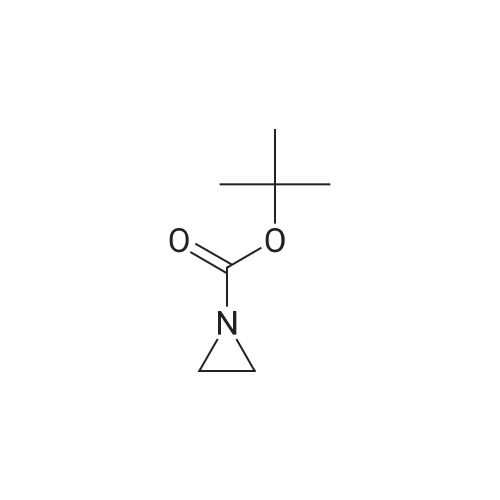
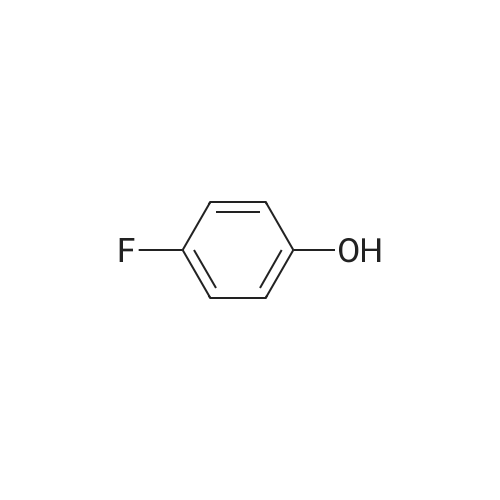
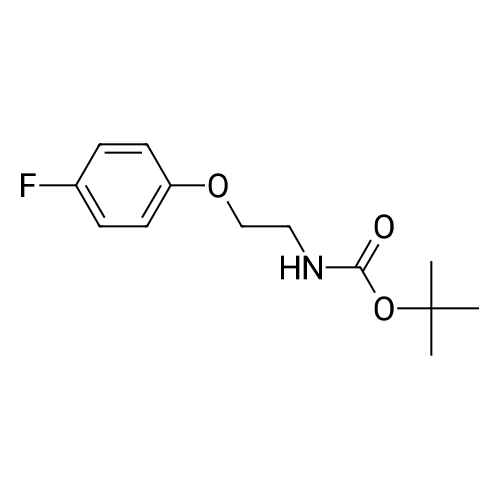
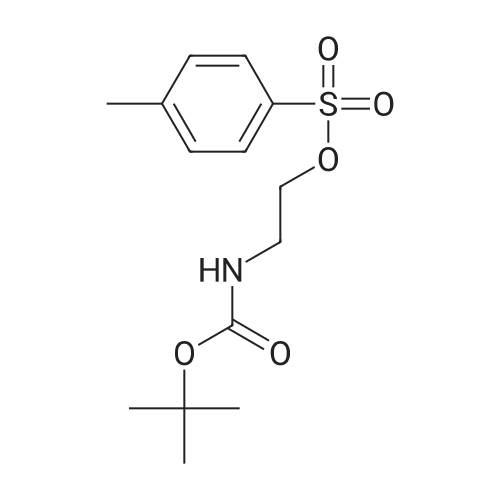
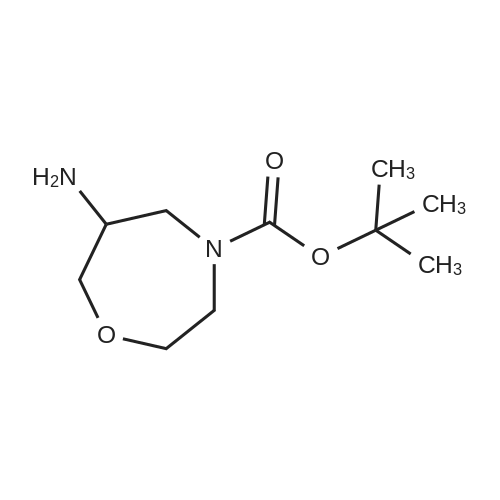
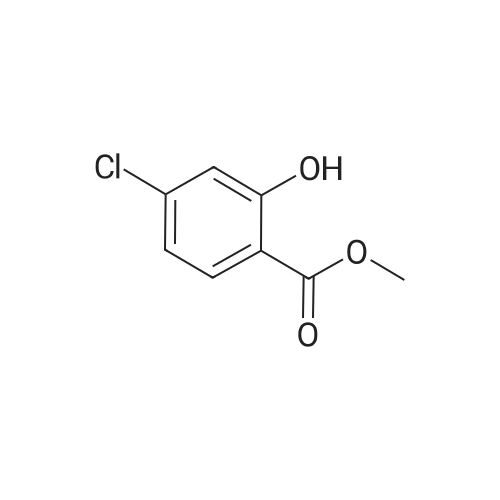
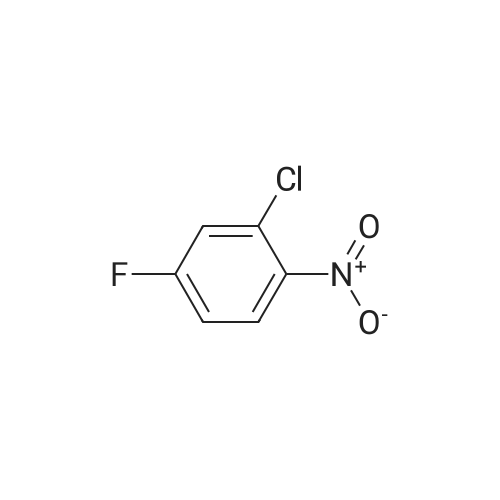
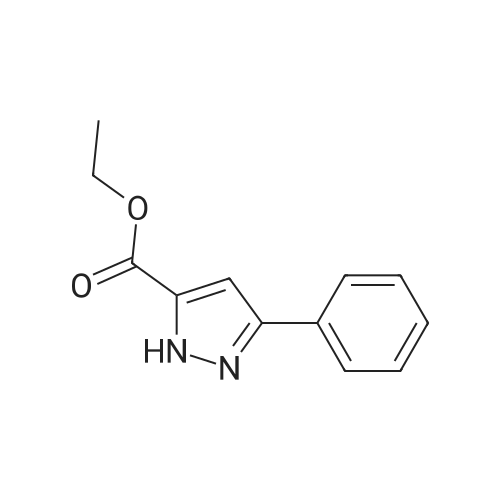
| 79% |
With triethylamine; In dichloromethane; at 20℃; |
To a mixture of tert-butyl 2-hydroxyethylcarbamate (6.4 g, 40 mmol) and Et3N (7.2 mL, 52 mmol) in DCM (50 mL) was added para-toluene sulfonylchloride, TsCl (8.4 g, 44 mmol). The mixture was stirred at room temperature (rt) overnight (ON). The reaction mixture was concentrated and the residue was partitioned between EA and water. The organic layer was separated, dried over Na2SO4, filtered and concentrated. The residue was purified by silica gel chromatography (PE/EA=5/1) to give 2-(tert-butoxycarbonylamino)ethyl 4-methylbenzenesulfonate (10.0 g, 79%) as white solid. |
| 79% |
With triethylamine; In dichloromethane; |
Preparation of toluene-4-sulfonic acid 2-tert-butoxycarbonylamino-ethyl ester. Tosyl chloride (1.50 g, 7.87 mmol) was added to a solution of (2-hydroxy-ethyl)-carbamic acid tert-butyl ester (0.84 g, 5.2 mmol) and Et3N (1.23 mL, 8.82 mmol) in CH2Cl2 (26 mL), and the solution was stirred at room temperature for 17 h. The solution was washed with H2O (15 mL), and the aqueous phase was extracted with CH2Cl2 (10 mL). The combined organic phases were dried (MgSO4) and concentrated in vacuo. Purification of the crude material by column chromatography on silica gel (20% EtOAc/hexanes) gave yellow crystals (1.29 g, 79%). 1H NMR (CDCl3) δ 1.41 (s, 9H), 2.45 (s, 3H), 3.38 (m, 2H), 4.07 (m, 2H), 4.82 (br s, 1H), 7.35 (d, 2H, J=8.1 Hz), 7.79 (d, 2H, J=8.1 Hz). |
| 73% |
With pyridine; at 0 - 20℃; |
N-(tert-Butyloxycarbonyl)-O-(4-methylphenylsulphonyl)ethanolamine. A stirred solution of N-(tert-butyloxycarbonyl)ethanolamine (16.1 g, 0.10 mol) in pyridine (50 ml) at 0 C was treated with 4-methylphenylsulphonyl chloride (19.1 g, 0.10 mol) and the mixture stirred and allowed to warm to room temperature overnight. Pyridine was removed in vacuo at room temperature and the residue partitioned between 2 M hydrochloric acid and dichloromethane. The organic layer was separated and the solvent removed in vacuo to afford the crude product as a pale yellow oil (23.0 g, 73%). 1H NMR (DMSO-d6) 7.78 (2H, d), 7.49 (2H, d), 7.01 (IH, t), 3.96 (2H, t), 3.12 (2H, m), 2.41 (3H, s), 1.31 (9H, s). |
| 61.3% |
With triethylamine; In dichloromethane; at 25℃; for 12h; |
To a mixture of tert-butyl (2-hydroxyethyl)carbamate (100 g, 620 mmol, 06.2 mL, 1 eq) and tri ethylamine (173 mL, 1.24 mol, 2 eq) in dichloromethane (1 L) was added 4- methylbenzenesulfonyl chloride (177 g, 930 mmol, 1.5 eq) in one portion at 25 Cunder nitrogen. The mixture was stirred at 25 C for 12 hr. The residue was poured into water (1 L) and stirred for 3 min. The aqueous phase was extracted with ethyl acetate (500 mL * 3). The combined organic phase was dried with anhydrous sodium sulfate, filtered and concentrated in vacuum. The residue was purified by column chromatography (silica, petroleum ether/ethyl acetate=l/O to 8/1) to afford the title compound (120 g, 61.3 % yield) as a white solid. ’H NMR (400 MHz, CDCh-d) 5 ppm 1 .39 (s, 9 H) 2.43 (s, 3 H) 3.36 (br d, 2 H) 4.05 (t, 2 H) 4.92 (br s, 1 H) 7.33 (d, 2 H) 7.77 (d, 2 H). |
| 50% |
With pyridine; at 20℃; for 12h; |
Will be purchased at Ruiouke Technology CAS No. 26690-80-2;Product No. 6, product number R0K11845-2 (5.00 g, 31.06 mmol) and TsCl (11.88 g, 62.12 mmol) were dissolved in 20 mL of pyridine,The reaction was complete at room temperature for 12 h, and the reaction was complete.The organic layer was dried over magnesium sulfate and concentrated. The crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 3: 1) to give white solid compound (7), and the residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 3: 1). (4.89 g, yield 50%). |
| 50% |
With pyridine; at 20℃; for 12h; |
raw materials (tert-butoxycarbonyl)aminoethanol (5.8 g, 31.06 mmo 1) and TsCl (11.88 g, 62 · 12 mmol) were dissolved in 20 mL of pyridine and stirred at room temperature for 12 h. TLC was complete. A portion of pyridine was removed by distillation under reduced pressure, water was added, and the mixture was extracted three times with ethyl acetate. The organic layer was dried over magnesium sulfate and concentratedThe crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 3: 1) to give white solid compound 2 (4.89 g, yield50 ) ο |
| 46% |
With pyridine; at 0℃; |
67B: N-Boc-2-Tosyl-ethylamine; Pyridine (1.5 mL, 18.5 mmol) was added to 2-(N-Boc-amino)-ethanol (280 mg, 1.7 mmol) at 0C. Tosyl chloride (1.65g, 8.7 mmol) was then added and the reaction was allowed to stir at 0C overnight. The resulting cloudy reaction was diluted with Et2O and washed with water, saturated NaHCOs and brine, dried over MgSC>4 and concentrated. The resulting white solid was purified using SiO2 with AcOEt/petroleum ether 20:80 to 30:70. A white solid was obtained (250 mg, 46%). NMR 1H (ppm, CDC13): 7.77 (d, f = 8.3 Hz, 2H), 7.33 (d, f = 8.0 Hz, 2H), 4.82 (br. s., 1H), 4.05 (t, J3 = 5.08 Hz, 2H), 3.38-3.33 (m, 2H), 2.43 (s,3H), 1.39(s,9H). |
| 46% |
With pyridine; at 0℃; |
67B: N-Boc-2-Tosyl-ethylamine; Pyridine (1.5 mL, 18.5 mmol) was added to 2-(N-Boc-amino)-ethanol (280 mg, 1.7 mmol) at 0 C. Tosyl chloride (1.65 g, 8.7 mmol) was then added and the reaction was allowed to stir at 0 C. overnight. The resulting cloudy reaction was diluted with Et2O and washed with water, saturated NaHCO3 and brine, dried over MgSO4 and concentrated. The resulting white solid was purified using SiO2 with AcOEt/petroleum ether 20:80 to 30:70. A white solid was obtained (250 mg, 46%). NMR 1H (ppm, CDCl3): 7.77 (d, J3=8.3 Hz, 2H), 7.33 (d, J3=8.0 Hz, 2H), 4.82 (br. s., 1H), 4.05 (t, J3=5.08 Hz, 2H), 3.38-3.33 (m, 2H), 2.43 (s, 3H), 1.39 (s, 9H). |
| 30.7% |
|
To a stirred solution of tert-butyl (2-hydroxyethyl)carbamate (step 1, 25.0 g, 155.0mmol, 1.0 eq) in DCM (250 ml) was added triethyl amine (46.9 g, 465.0 mmol, 3.0 eq) andDMAP (1.89 g, 15.5 mmol, 0.1 eq). The reaction mixture was stirred at room temperature for10 minutes and then para-Toluenesulfonylchloride (32.4 g, 170.0 mmol, 1.1 eq) was added at0 C. The reaction mixture was stirred at room temperature for about 14 hours. TLC indicatedstarting material was consumed and the desired product was observed. The reaction mixturewas diluted with water (250 ml) and extracted with DCM (2x250 ml). The combined organicextracts were dried over sodium sulfate, filtered and evaporated under reduced pressure. The residue was purified by silicagel column chromatography by using 20% EtOAc in hexane as an eluent to obtain the desired product (15.0 g, 30.7% yield) as a white solid. ‘H NMR (300 MHz, CDC13): ppm 7.79 (d, J= 8.1 Hz, 2H), 7.35 (d, J= 8.1 Hz, 2H), 4.86 (s, 1H), 4.06 (t,J = 4.8 Hz, 2H), 3.38 (t, J = 5.1 Hz, 2H), 2.45 (s, 3H), 1.40 (s, 9H); ESI-MS: mlz 338.08 (M+Na)t |
|
With dmap; triethylamine; In dichloromethane; |
b) Synthesis of 2-tert-butoxycarbonylaminoethyl 4-toluenesulfonate A mixture of 2-tert-butoxycarbonylaminoethanol (32.7 g, 203 mmol), triethylamine (34 ml), p-toluenesulfonyl chloride (38.7 g, 203 mmol), 4-dimethylaminopyridine (0.10 g) and dichloromethane (500 ml) was stirred at 0 C. for 4 hours. The reaction mixture was washed successively with water, 1N hydrochloric acid, a saturated aqueous sodium hydrogencarbonate solution and a saturated aqueous sodium chloride solution. The organic layer was dried over anhydrous magnesium sulfate and the solvent was distilled off under reduced pressure. The resulting residue was purified by a silica gel column chromatography (eluent:ethyl acetate/n-hexane= 1/5) to obtain 48.2 g of 2-tert-butoxycarbonylaminoethyl 4-toluenesulfonate. 1 Hnmr (CDCl3) δ: 1.41(9H, s), 2.44(3H, s), 3.38(2H, dt, J=5.6 Hz, 5.6 Hz), 4.07(2H, t, J=5.3 Hz), 4.87(1H, br-s), 7.35(2H, dd, J=0.7 Hz, 8.6 Hz), 7.78(2H, ddd, J=2.0 Hz, 2.0 Hz, 8.3 Hz). |
|
With dmap; In dichloromethane; at 0 - 20℃; for 15h; |
(Reference Example 7) Synthesis of 2-((6-methylpyridin-3-yl)oxy)ethylamine dihydrochloride To a solution of tert-butyl N-(2-hydroxyethyl)carbamate (49.9 g, 310 mmol) and 4-dimethylaminopyridine (42 g, 340 mmol) in dichloromethane (500 mL) was slowly added tosyl chloride (59 g, 310 mmol) under ice-cooling. The mixture was allowed to cool to room temperature, stirred for 15 hr, washed with water and saturated brine, and concentrated to give 2-((tert-butoxycarbonyl)amino)ethyl 4-methyl benzenesulfonate (98 g). 66 g (209 mmol) of these was dissolved in DMF (500 mL), 5-hydroxy-2-methylpyridine (22.8 g, 209 mmol) and cesium carbonate (102 g, 314 mmol) were added, and the mixture was stirred at 100C for 2 hr. The reaction mixture was allowed to cool to room temperature, poured into cool water, and extracted with ethyl acetate three times. The organic layer was washed with saturated brine, and concentrated to give crystals (35 g). The crystals were washed with tert-butyl-methyl ether to give tert-butyl (2-((6-methylpyridin-3-yl)oxy)ethyl)carbamate (24.5 g) as white crystals. The crystals were treated with 2N-HCl/Dioxane solution (140 ml) at room temperature, and the obtained insoluble substance was collected by filtration to give the object compound of 2-((6-methylpyridin-3-yl)oxy)ethylamine dihydrochloride (17.6 g). 1H-NMR(300MHz, DMSO-d6)δ(ppm) : 8.49(d,1H), 8.42(br-s,2H), 8.05(dd,1H), 7.78(d,1H), 4.40(t,2H)3.23-3.13(m,2H) |
|
With triethylamine; In dichloromethane; at 20℃;Cooling with ice; |
step 1. N-tert-Boc-ethanolamine (14 g, 87 mmol), TEA (27.3 mL, 195 mmol) and DCM (180 mL) were combined in a 500 mL round bottom flask and cooled in an ice bath. p-Toluenesulfonyl chloride (23.1 g, 122 mmol), dissolved in DCM (180 mL), was added and the ice bath removed. The mixture was stirred at RT overnight. The mixture was washed with water, brine and dried (Na2SO4), filtered and concentrated in vacuo to afford 2-(tert-butoxycarbonylamino)ethyl 4-methylbenzenesulfonate (26 g, crude) as a light yellow oil. LCMS (ESI): m/z=338 (M+Na)+. |
|
With pyridine; at -10 - 4℃; for 96h; |
[0839] To a solution oftert-butyl(2-hydroxyethyl)carbamate(0.960 mL, 6.08 mmol) in pyridine (25 ml) was added4-methylbenzene-1-sulfonyl chloride (2.341 g, 12.16 mmol)at -1 oo C. The solution was stirred at -1 oo C. for 40 min. Thereaction mixture was stored in a fridge at 4 C. for 4 days. Itwas poured into ice water and extracted with ethyl acetate.The combined organic layers were washed aqueous citric acid( 10% ), dried using MgSO 4 , filtered and evaporated to give thetitle compound as a yellow oil. (UPLC-MS 3) ESI-MS 316.2[M+Ht. 1HNMR(600MHz, CDCI3 ) o7.79 (d, 2H), 7.36 (d,2H), 4.84 (s, lH), 4.06 (t, 2H), 3.41-3.35 (m, 2H), 2.45 (s,3H), 1.40 (s, 9H). |
|
With pyridine; at -10 - 4℃; for 111.84h; |
To a solution of tert-butyl (2-hydroxyethyl)carbamate (0.960 mL, 6.08 mmol) in pyridine (25 ml) wasadded 4-methylbenzene-1-sulfonyl chloride (2.341 g, 12.16 mmol) at -10 C. The solution wasstirred at -10 C for 40 mm. The reaction mixture was stored in a fridge at 4 C for 4 days. It was poured into ice water and extracted with ethyl acetate. The combined organic layers were washed aqueous citric acid (10%), dried using MgSO4, filtered and evaporated to give the title compound as a yellow oil. (UPLC-MS 3) ESI-MS 316.2 [M+H]. 1H NMR (600 MHz, CDCI3) 5 7.79 (d, 2H), 7.36(d, 2H), 4.84 (s, I H), 4.06 (t, 2H), 3.41 - 3.35 (m, 2H), 2.45 (s, 3H), 1.40 (s, 9H). |
| 287 g |
With triethylamine; In toluene; at 40℃; for 6h; |
In a reaction flask, (tert-butoxycarbonyl)-ethanolamine (121.40 g, 0.75 mol), toluene (600.00 ml), triethylamine (124.46 g, 1.23 mol) were loaded, and the temperature was raised to about 40 C. A solution of p-toluenesulphonyl chloride (214.48 g, 1.12 mol) in toluene (600.00 ml) was added and the reaction mixture was maintained under these conditions for about six hours. Once the reaction is finished, the temperature was cooled to about 20 C., water (200.00 ml) was added and the organic phase was washed with water (1*200.00 ml), with an aqueous solution of 2N hydrochloric acid (1*240.00 ml) and with water again (2*200.00 ml), the collected organic phases were concentrated till residue through vacuum distillation to give 287.00 g of 2-[(tert-butoxycarbonyl)amino]ethyl 4-methylbenzensulphonate. 1H-NMR (CDCl3, 300 MHz): δ 7.77 (d, 2H), 7.31 (d, 2H), 4.04 (t, 2H), 3.35 (t, 2H), 2.42 (s, 3H), 1.41 (s, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping