Alternatived Products of [ 2657-87-6 ]
Product Details of [ 2657-87-6 ]
| CAS No. : | 2657-87-6 |
MDL No. : | MFCD00036097 |
| Formula : |
C12H12N2O
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | ZBMISJGHVWNWTE-UHFFFAOYSA-N |
| M.W : |
200.24
|
Pubchem ID : | 75871 |
| Synonyms : |
|
Application In Synthesis of [ 2657-87-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 2657-87-6 ]
- 1
-
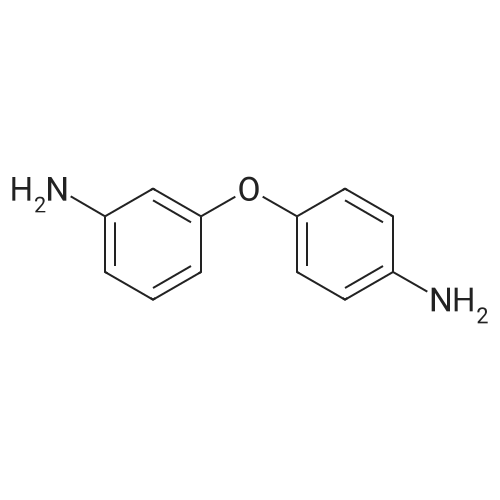 [ 2657-87-6 ]
[ 2657-87-6 ]

-
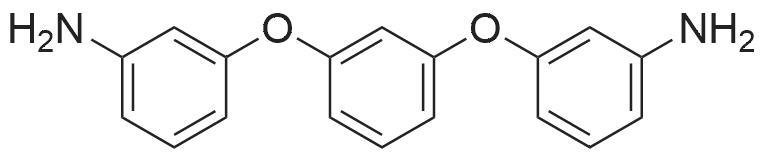 [ 10526-07-5 ]
[ 10526-07-5 ]

-
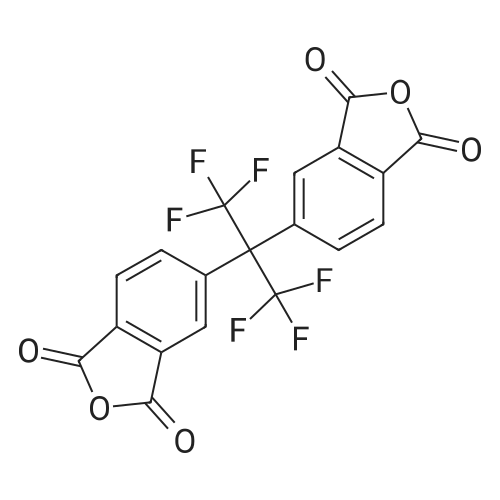 [ 1107-00-2 ]
[ 1107-00-2 ]

-
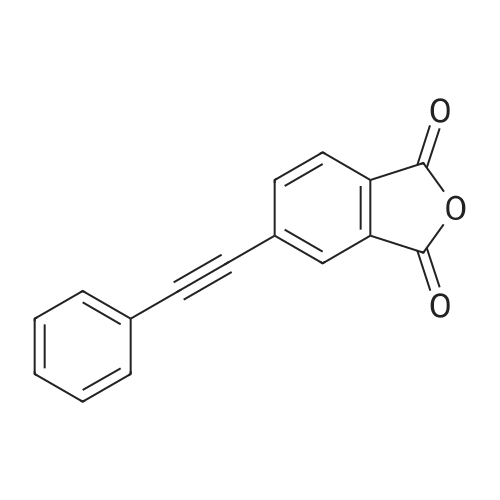 [ 119389-05-8 ]
[ 119389-05-8 ]

-
polymer, Mn 3491 Da, Mw 6667 Da, PDI 1.91; monomer(s): 4,4\-(hexafluoroisopropylidene)diphthalic anhydride; 3,4\-diaminodiphenyl ether; 1,3-bis(3-aminophenoxy)benzene; 4-(phenylethynyl)phthalic anhydride
[ No CAS ]
- 2
-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 10526-07-5 ]
[ 10526-07-5 ]

-
 [ 1107-00-2 ]
[ 1107-00-2 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
polymer, Mn 5367 Da, Mw 11056 Da, PDI 2.06; monomer(s): 4,4\-(hexafluoroisopropylidene)diphthalic anhydride; 3,4\-diaminodiphenyl ether; 1,3-bis(3-aminophenoxy)benzene; 4-(phenylethynyl)phthalic anhydride
[ No CAS ]
- 3
-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 10526-07-5 ]
[ 10526-07-5 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
4,4-(2,2,2-trifluoro-1-phenylethylidene)bis(phthalic anhydride)
[ No CAS ]

-
polymer, Mn 3215 Da, Mw 5855 Da, PDI 1.82; monomer(s): 4,4\-(2,2,2-trifluoro-1-phenylethylidene)diphthalic anhydride; 3,4\-diaminodiphenyl ether; 1,3-bis(3-aminophenoxy)benzene; 4-(phenylethynyl)phthalic anhydride
[ No CAS ]
- 4
-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 10526-07-5 ]
[ 10526-07-5 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
4,4-(2,2,2-trifluoro-1-phenylethylidene)bis(phthalic anhydride)
[ No CAS ]

-
polymer, Mn 5301 Da, Mw 11769 Da, PDI 2.16; monomer(s): 4,4\-(2,2,2-trifluoro-1-phenylethylidene)diphthalic anhydride; 3,4\-diaminodiphenyl ether; 1,3-bis(3-aminophenoxy)benzene; 4-(phenylethynyl)phthalic anhydride
[ No CAS ]
- 5
-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 10526-07-5 ]
[ 10526-07-5 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
4,4-(2,2,2-trifluoro-1-phenylethylidene)bis(phthalic anhydride)
[ No CAS ]

-
polymer; monomer(s): 4,4\-(2,2,2-trifluoro-1-phenylethylidene)diphthalic anhydride; 3,4\-diaminodiphenyl ether; 1,3-bis(3-aminophenoxy)benzene; 4-(phenylethynyl)phthalic anhydride
[ No CAS ]
- 6
-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
4,4-(2,2,2-trifluoro-1-phenylethylidene)bis(phthalic anhydride)
[ No CAS ]

-
polymer, Mn 5207 Da, Mw 10830 Da, PDI 2.07; monomer(s): 4,4\-(2,2,2-trifluoro-1-phenylethylidene)diphthalic anhydride; 3,4\-diaminodiphenyl ether; 4-(phenylethynyl)phthalic anhydride
[ No CAS ]
- 7
-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 2420-87-3 ]
[ 2420-87-3 ]

-
 [ 10526-07-5 ]
[ 10526-07-5 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
polymer, Mn 3491 Da, Mw 6796 Da, PDI 1.94; monomer(s): 3,3\,,4,4\-biphenylenetetracarboxylic dianhydride; 3,4\-diaminodiphenyl ether; 1,3-bis(3-aminophenoxy)benzene; 4-(phenylethynyl)phthalic anhydride
[ No CAS ]
- 8
-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 2420-87-3 ]
[ 2420-87-3 ]

-
 [ 10526-07-5 ]
[ 10526-07-5 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
polymer, Mn 8383 Da, Mw 14496 Da, PDI 1.73; monomer(s): 3,3\,,4,4\-biphenylenetetracarboxylic dianhydride; 3,4\-diaminodiphenyl ether; 1,3-bis(3-aminophenoxy)benzene; 4-(phenylethynyl)phthalic anhydride
[ No CAS ]
- 9
-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 2420-87-3 ]
[ 2420-87-3 ]

-
 [ 10526-07-5 ]
[ 10526-07-5 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
polymer; monomer(s): 3,3\,,4,4\-biphenylenetetracarboxylic dianhydride; 3,4\-diaminodiphenyl ether; 1,3-bis(3-aminophenoxy)benzene; 4-(phenylethynyl)phthalic anhydride
[ No CAS ]
- 10
-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 2420-87-3 ]
[ 2420-87-3 ]

-
 [ 10526-07-5 ]
[ 10526-07-5 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
polymer; monomer(s); 3,3',4,4'-biphenyltetracarboxylic dianhydride; 3,4'-oxydianiline; 1,3-bis(3-aminophenoxy)benzene; 4-phenylethynylphthalic anhydride
[ No CAS ]
- 11
-
 [ 1823-59-2 ]
[ 1823-59-2 ]

-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
poly(4-phenyl-ethynyl-phthalic anhydride)-co-(3,4'-oxydianiline)
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In 1-methyl-pyrrolidin-2-one; at 100 - 350℃; for 1h; |
Example 5; Synthesis of imide oligomer by Using 4-phenyl ethynyl phthalic anhydride Obtained in Example 3 and Comparative Examples 1 to 3 as Terminal End Groups; In accordance with the method as described in Non-Patent Document No. 2, a solution of an amide-acid oligomer having an average molecular weight of about 9,000 was prepared from the 4-phenyl ethynyl phthalic anhydrides obtained in Example 3 and Comparative Examples 1 to 3, and 3,4'-oxydianiline and an N-methylpyrrolidone solution of 4,4'-oxydiphthalic anhydride. The thus-prepared amide-acid oligomer was centrifuged, applied, dried and subjected to thermal treatments for one hour at 100° C., 225° C. and 350° C. in this order, to thereby obtain films of cross-linked imide oligomer. On the other hand, toluene was added to an N-methylpyrrolidone solution of the amide-acid oligomer and, the mixture was subjected to the steps of azeotropic dehydration, cooling, filtration, rinsing with water and methanol in this order, and drying, to thereby isolate an imide oligomer. Tg and kinetic properties at 23° C. of each film prepared in accordance with the above-described method corresponding to 4-phenyl ethynyl phthalic anhydrides obtained in Example 3 and Comparative Examples 1 to 3 were measured in accordance with a method as specified in ASTM D882 and, also a temperature at which 5percent by mass of the imide oligomer was reduced was measured by using a thermobalance. These results are shown in Table 1. TABLE 1 Production method for 4-phenyl Film of cross-linked Temper- ethynyl imide oligomer (23° C.) ature of phthalic Tensile Elastic Elongation 5percent mass anhydride Tg strength modulus at break reduction Example 3 252° C. 122.2 MPa 3.0 GPa 55percent 518° C. Comparative 250° C. 120.1 Mpa 2.6 GPa 31percent 514° C. Example 1 Comparative 252° C. 118.8 MPa 2.8 GPa 36percent 511° C. Example 2 Comparative 251° C. 119.7 MPa 2.7 GPa 36percent 514° C. Example 3 |
- 12
-
 [ 2657-87-6 ]
[ 2657-87-6 ]

-
 [ 2420-87-3 ]
[ 2420-87-3 ]

-
 [ 119389-05-8 ]
[ 119389-05-8 ]

-
C128H82N8O28
[ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping










