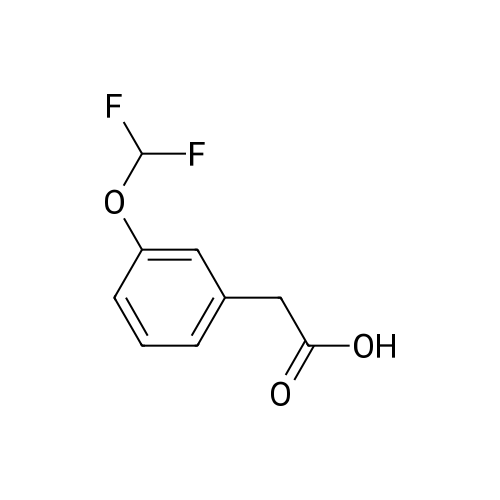
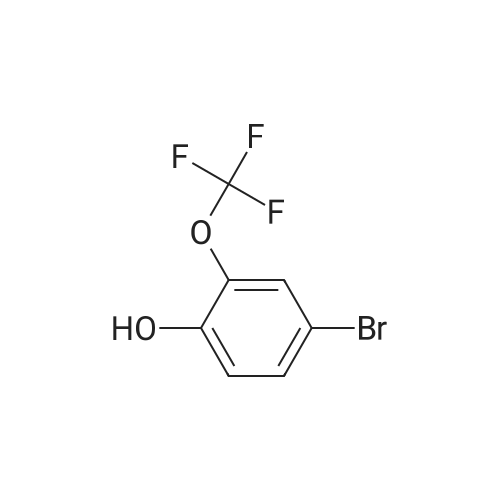
| 34% |
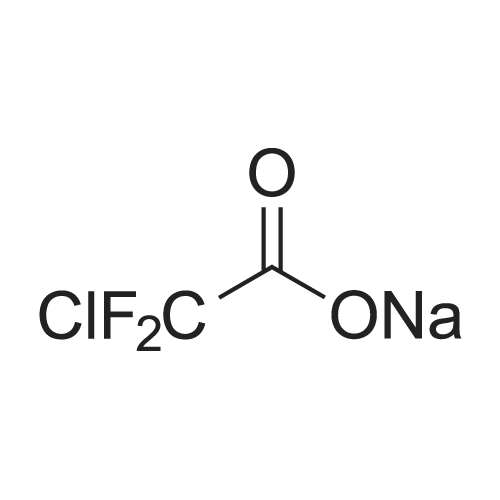
With potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In 1,4-dioxane; at 100℃; for 21h;Inert atmosphere; |
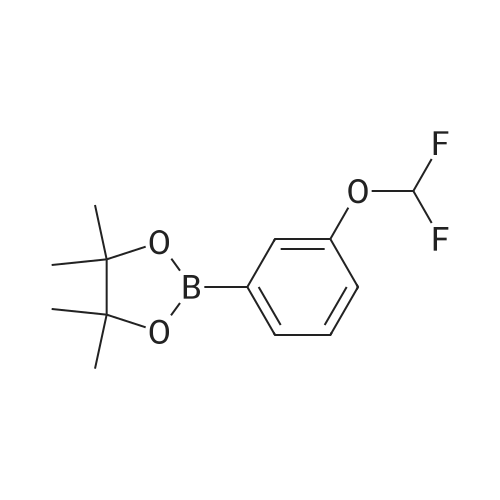
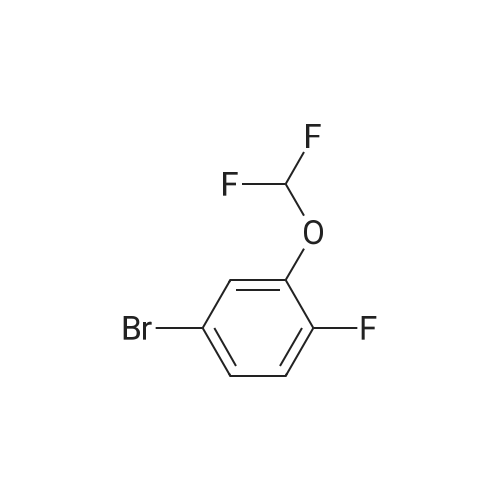
2-(3-(Difluoromethoxy)phenyl)-4,4,5,5-tetramethyl-1,3,2- dioxaborolane (T18.2). A stirred mixture of T18.1 (1.00 g, 4.50 mmol), bis(pinacolato)diboron (1.26 g, 4.95 mmol), potassium acetate (1.34 g, 13.70 mmol), and dichloro[l,l'-bis(diphenylphosphino)ferrocene]dichloride palladium(II) DCM adduct (0.17 g, 0.23 mmol) in dry 1,4-dioxane (10.0 mL)was purged three times with argon and placed under vacuum three times. The mixture was heated to 100C and monitored with LC-MS and TLC. After 21 hours, the reaction was cooled to room temperature and then filtered through Celite filter aid. The organic solvent was removed under reduced pressure, and the residue was purified on silica gel (0-10% EtOAc in hexanes) to yield T18.2 as a colorless oil (0.41 g, 34%). 1H NMR (400 MHz, CDCl3) delta ppm 7.67 (1 H, d, J=7.4 Hz), 7.56 (1 H, d, J=2.3 Hz), 7.41 (1 H, m), 7.22 (1 H, dd, J=7.8, 2.3 Hz), 6.73 (1H, t, J= 74 Hz), 1.36 (12 H, s). |
| 34% |
With potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In 1,4-dioxane; at 100℃; for 21h;Inert atmosphere; |
2-(3-(Difluoromethoxy)phenyl)-4,4,5,5-tetramethyI-1,3,2- dioxaborolane (T7.2). A stirred mixture of T7.1 (1.00 g, 4.50 mmol), bis(pinacolato)diboron (1.26 g, 4.95 mmol), potassium acetate (1.34 g, 13.70 mmol), and dichloro[l,r-bis(diphenylphosphino)ferrocene]dichloride palladium(II) DCM adduct (0.17 g, 0.23 mmol) in dry 1,4-dioxane (10.0 mL)was purged three times with argon and placed under vacuum three times. The mixture was heated to 100C and monitored with LC-MS and TLC. After 21 hours, the reaction was cooled to room temperature and then filtered through Celite filter aid. The organic solvent was removed under reduced pressure, and the residue was purified on silica gel (0-10% EtOAc in hexanes) to yield T7.2 as a colorless oil (0.41 g, 34%). 1H NMR (400 MHz, CDCl3) delta ppm 7.67 (1 H, d, J=7.4 Hz), 7.56 (1 H, d, J=2.3 Hz), 7.41 (1 H, m), 7.22 (1 H, dd, J=7.8, 2.3 Hz), 6.73 (1H, t, J= 74 Hz), 1.36 (12 H, s). |
|
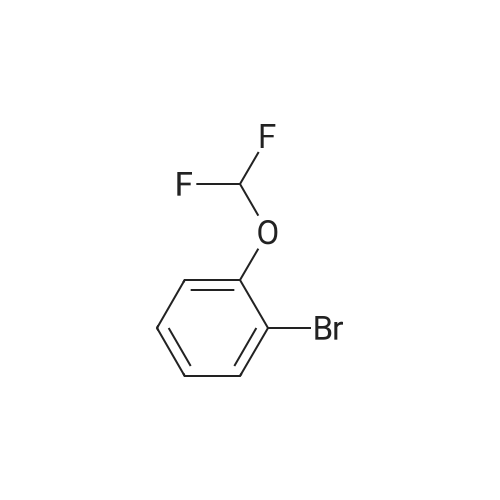
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,4-dioxane; at 90℃; for 21h;Inert atmosphere; |
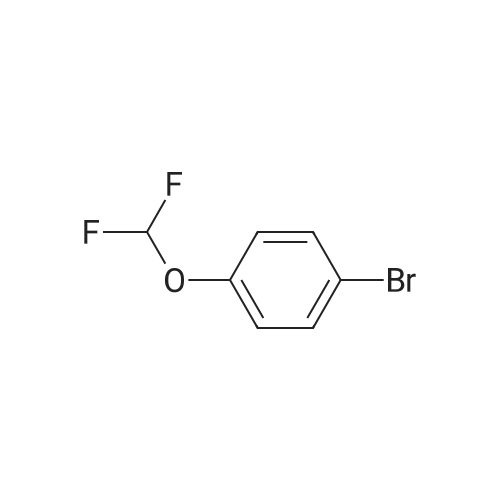
A stirred mixture of l-bromo-3-(difluoromethoxy)benzene (0.56 mL, 4 mmol), bis(pinacolato)diboron (1.21 g, 4.8 mmol), l,l'-bis(diphenylphosphino)ferrocene- palladium dichloride (0.32 g, 0.4 mmol), and potassium acetate (1.18 g, 12 mmol) in dry 1,4-dioxane (10.0 mL) was purged three times with argon and placed under vacuum three times. The mixture was heated to 90 C and monitored with LC- MS and TLC. After 21 h, the reactions were cooled to rt then filtered through celite. The organic solvent was removed under reduced pressure, and the residue was purified on silica gel (0-10% EtOAc in hexanes) to yield a yellow liquid as 2- (3-(difluoromethoxy)phenyl)-4,4,5,5-tetramethyl-l ,3,2-dioxaborolane. 1H NMR (400 MHz, CDC13) delta ppm 7.66 (1 H, d, J=7.2 Hz), 7.54 (1 H, d, J=2.3 Hz), 7.38(1 H, t, J=7.7 Hz), 7.25 (1 H, m), 6.54 (1 H, t), 1.36 (12 H, s). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping