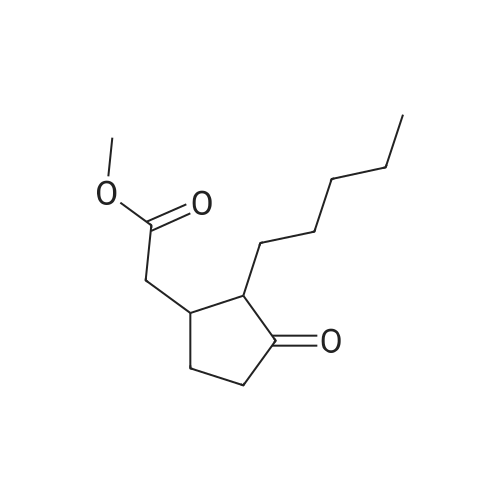
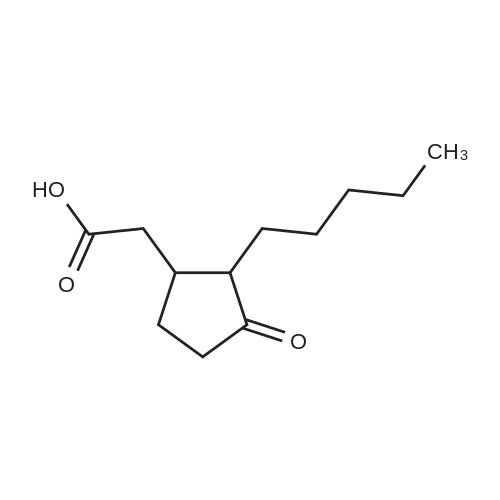
| 28% |
Stage #1: With sodium methylate In methanol at 0℃; for 5 h; Michael addition
Stage #2: at 215℃; for 4 h; |
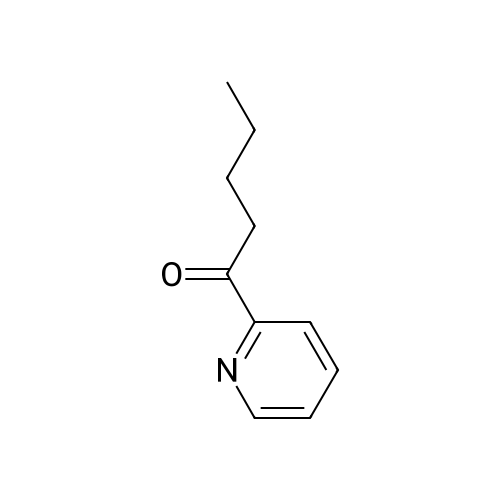
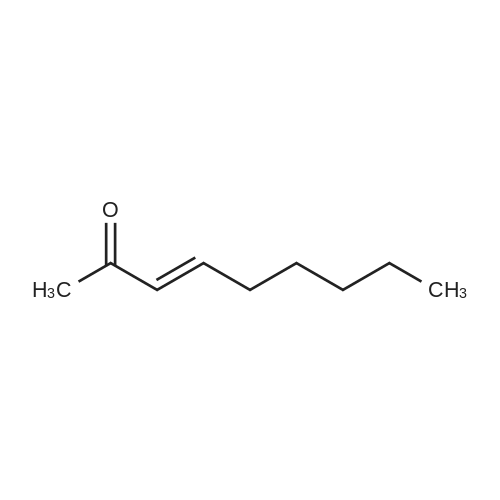
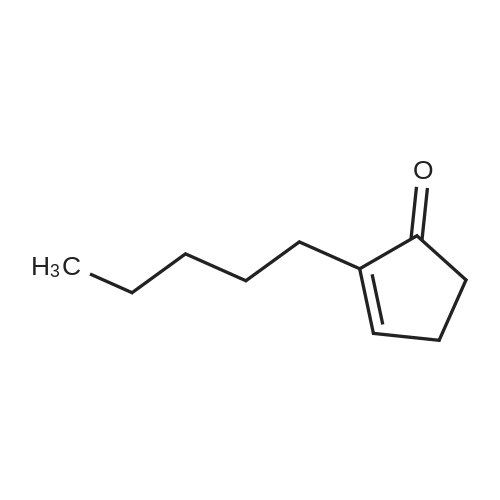
The reaction in Example 1 was carried out twice, the product was distilled to recover cyclopentanone and water, then 0.0206 mol oxalic acid was added to 1.01 mol 2-(1-hydroxy-n-pentyl)cyclopentanone and 0.022 mol 2-pentylidene cyclopentanone from the product, and the mixture was reacted at 120°C. The reaction mixture contained 141 g (0.93 mol) 2-pentylidene cyclopentanone. Its filtered product was dissolved in 153 g n-butanol and heated at 130°C, and then a mixture of 14.5 g (0.15 mol) 3-picoline and 10.5 g (0.1 mol) 35percent hydrochloric acid was added dropwise at the same temperature over 30 minutes. Thereafter, the mixture was stirred at the same temperature for 3.5 hours. After the reaction was finished, the mixture was cooled to room temperature and neutralized with an aqueous sodium hydroxide solution, and the organic layer was analyzed. The result indicated that the reaction mixture contained 118 g 2-pentyl-2-cyclopentenone. The yield in this isomerization reaction was 83percent. From this reaction mixture, 95 g (0.6 mol) 2-pentyl-2-cyclopentenone was purified. Separately, 118 g (0.9 mol) dimethyl malonate was dissolved in 38 g anhydrous methanol in a nitrogen atmosphere and then cooled to 0°C, and 6.5 g (0.036 mol) sodium methoxide (30percent methanol) was added thereto. 95 g (0.6 mol) 2-pentyl-2-cyclopentenone obtained above was added dropwise thereto at 0°C over 2 hours. Thereafter, the mixture was stirred at the same temperature for 3 hours. Thereafter, the unreacted dimethyl malonate was distilled away under reduced pressure, whereby 160 g Michael addition product was obtained. The Michael addition product obtained above was added to a reaction device equipped with a distillation tube and then heated at 215°C, and water was added dropwise at a rate of 3.2 g/h (2percent/h). By adding water dropwise, the mixture was reacted for 4 hours at 215°C while generated carbon dioxide and methanol were distilled away. After the reaction was finished, 123 g methyl 3-oxo-2-pentylcyclopentylacetate was obtained in 126 g crude product. The yield in the whole process was 60percent. The crude product was refined by distillation to give methyl 3-oxo-2-pentylcyclopentylacetate having a fruity jasmine-like aroma, which was also excellent as a perfume material. The reaction in Comparative Example 1 was carried out 3 times, the product was distilled to recover cyclopentanone and water, then 0.0206 mol oxalic acid was added to 1.11 mol 2-(1-hydroxy-n-pentyl)cyclopentanone and 0.012 mol 2-pentylidene cyclopentanone from the product, and the mixture was reacted at 120°C. Thereafter, the reaction was carried out in the same manner as in Example 7, to give methyl 3-oxo-2-pentylcyclopentylacetate. As a result, the yield in the whole process was 28percent. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping