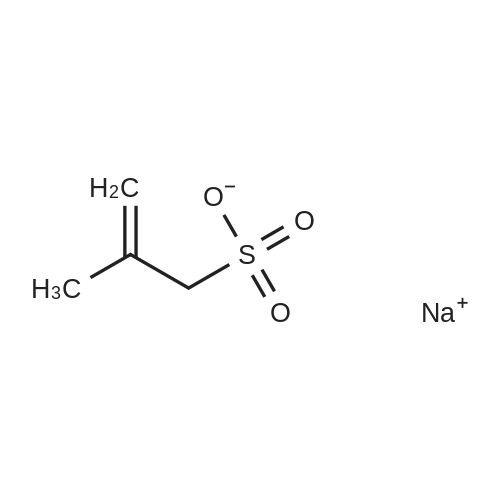
Comparison of Low-Density Lipoprotein Oxidation by Hydrophilic O(3P)-Precursors and Lipid-O(3P)-Precursor Conjugates
Maness, Peter F.
;
Cone, Grant W.
;
Ford, David A.
, et al.
Photochem. Photobiol.,2023,99(6):1412-1419.
DOI:
10.1111/php.13803
PubMed ID:
36943169
More
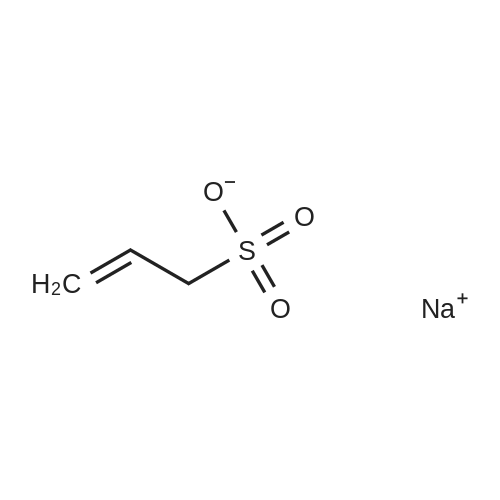
Abstract: Lipid oxidation by reactive oxygen species (ROS) provide several different oxidation products that have been implicated in inflammatory responses. Ground state at. oxygen [O(3P)] is produced by the photodeoxygenation of certain heterocyclic oxides and has a reactivity that is unique from other ROS. Due to the reactive nature of O(3P), the site of O(3P)-generation is expected to influence the products in heterogenous solutions or environments. In this work, the oxidation of low-d. lipoprotein (LDL) by lipids with covalently bound O(3P)-photoprecursors was compared to more hydrophilic O(3P)-photoprecursors. Lipid oxidation products were quantified after Bligh-Dyer extraction and pentafluorobenzyl bromide (PFB) derivatization by GC-MS. Unlike the more hydrophilic O(3P)-photoprecursors, the oxidation of LDL during the irradiation of lipid-(O3P)-photoprecursor conjugates showed little quenching by the addition of the O(3P)-scavenging sodium allyl sulfonate. This indicated that lipophilic O(3P)-photoprecursors are expected to generate lipid oxidation products where other more hydrophilic O(3P)-photoprecursors could be quenched by other reactive groups present in solution or the environment.
Purchased from AmBeed:
2495-39-8
-Precursors and Lipid-O(3P)-Precursor Conjugates.png)
Lipid Coupled Dibenzothiophene?S-Oxide Derivative as a Site Directed and Unquenchable Source of O(3P)
Grant W. Cone, B.S.
;
Saint Louis University,2023.
More
Abstract: Reactive oxygen species (ROS) and the understanding of how they influence cells provides insights onto how biological organisms react during oxidative stress caused by disease that plague humans and animals alike. In durations of high oxidative stress mediated by ROS, cells ultimately perish and can cause lasting effects on an organism. With such knowledge and the use of medicine, drugs are produced to aid therapies devised to combat the damage done by release of ROS in cells. While ROS have previously been extensively studied, more recent studies elucidated the existence and production of atomic oxygen, the smallest diradical yet known. Atomic oxygen or triplet oxygen with the term symbol 3P [O (3 P)], an extremely short lived and reactive species, produces a unique profile of products comparatively to other ROS and the usage of producing O (3 P) in cells is largely unknown and the benefits of doing so unrealized. The lack of knowledge comparative to other ROS is due to O (3 P) being a rare, if not unlikely, species produced in or by an organism. Currently, species with a Dibenzothiophene S-Oxide (DBTO) moiety are the predominate producers of O (3 P) utilizing photochemical processes. Previously, the production of O (3 P) in cells provided a “shotgun” approach where when incorporated into cells and DBTO’s subsequently photolyzed provided unique oxidation products, however, the usefulness of the technique provided to lack control necessary to provide breakthroughs in useful technology. Synthesizing a novel DBTO, one coupled to a lipid (phosphatidylcholine or PC), L-α-Phosphtidyl-N, N-dimethylethanolamine-N-2-methyldibenzothiophene S-oxide (PC-N-DBTO), provides a means to control the placement of O (3 P) production by predominately incorporating into membranes. Investigations into the usefulness of the novel technique of DBTO incorporation, completed by O (3 P) production in low density lipoprotein (LDL), shown that the unique oxidative product profile stayed intact. Furthermore, the investigation shown that compared to other techniques of incorporation, when the process utilizes PC-N-DBTO photolysis in LDL, that the process is nearly unquenchable using known O (3 P) quenching agent allylsufonate. Additionally, the examination provided evidence that isolation of DBTO to a specific site provides sufficient sequestering for studying the effects of O (3 P) production as a site directed approach.
Purchased from AmBeed:
2495-39-8
Development of Redox Organics for Aqueous Flow Batteries
Wu, Min
;
Harvard University,2021.
More
Abstract: The cost of solar and wind electricity has dropped so precipitously that the main barrier to their widespread adoption is their intrinsic intermittency. Aqueous organic redox flow batteries (AORFBs) provide a potentially cost-effective energy storage option to this end. However, many challenges remain in advancing AORFBs, particularly the synthetic cost and long-term stability of redox-active organic molecules.
Starting from inexpensive precursor, we developed a new synthetic route for extremely stable anthraquinone negative electrolyte (negolyte) active species. Furthermore, we demonstrated that high pH suppresses the decomposition of anthraquinone negolyte, leading to a capacity fade rate of 0.7%/year, representing one of the lowest capacity fade rates, in the absence of rebalancing techniques, of any flow battery ever published-organic or inorganic. Additionally, we reported an in-situ electrochemical oxidation method to decrease the synthetic cost.
To further address the cost challenge, we developed a one-pot, green, and scalable approach to synthesize a highly water-soluble and potentially low-cost anthraquinone. By a simple functionalization, the volumetric capacity and cycle life of this anthraquinone improved more than one order of magnitude. Additionally, simulation regarding state of charge, cut-off voltage, and cut-off current density provides guidance on how to measure the capacity fade rate more accurately.
In the final part, we developed the first anthraquinone negolyte species with a low capacity fade rate and a redox potential below -0.6 V vs. SHE at pH 14. The anthraquinone was synthesized from inexpensive precursors with a one-step N-alkylation method. Therefore, the mass production cost might be very low. The anthraquinone exhibited a high capacity fade rate of 2.6%/day at neutral pH, but the capacity fade rate decreased to 0.025%/day at pH 14, making it one of the most stable redox organic molecules ever reported. The substantial difference in anthraquinone cycling stability at different pH values was explained with thermodynamics.
Purchased from AmBeed:
2495-39-8


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping


-Precursors and Lipid-O(3P)-Precursor Conjugates.png)