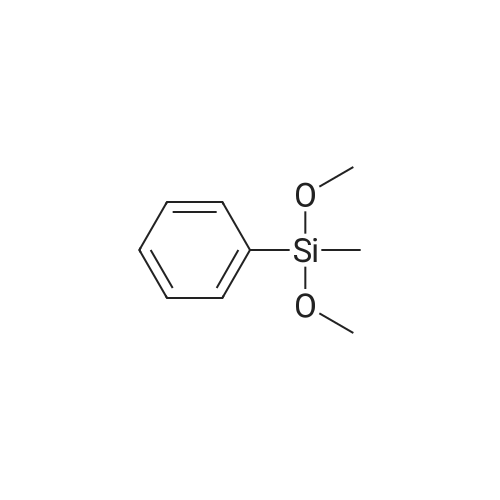
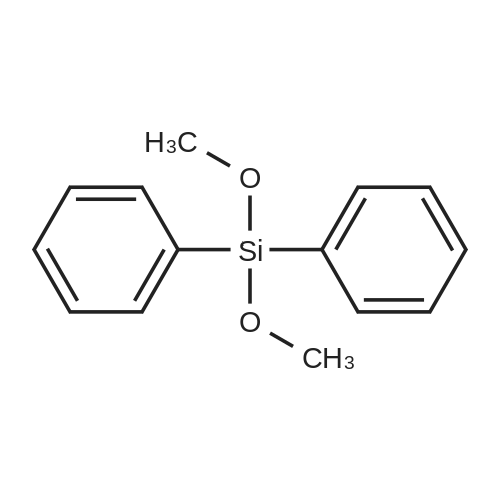
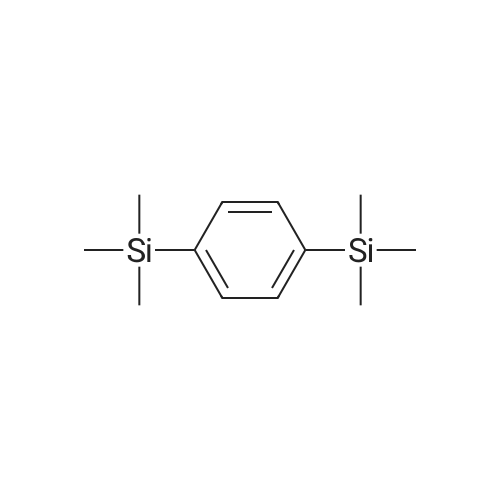
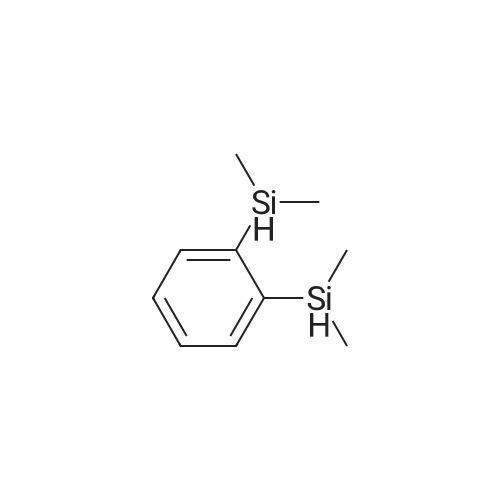
| 98% |
With magnesium; In tetrahydrofuran; at 50 - 85℃; for 16h;Inert atmosphere; |
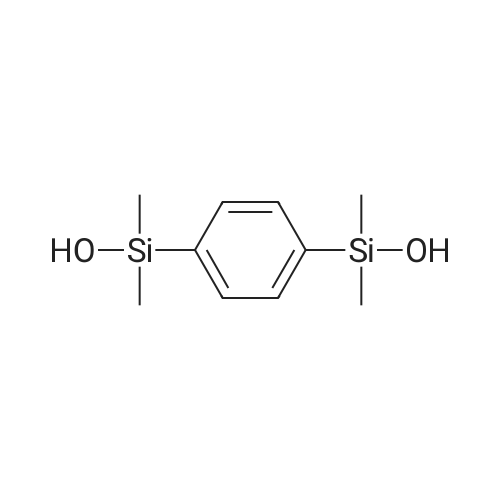
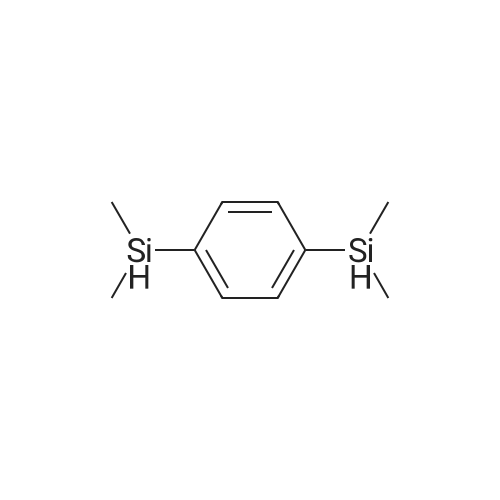
A three-neck round bottom flask is set up with a reaction device on which a feeding pipe and a return pipe are installed. 6.1 g (0.25 moles) of magnesium metal was weighed and placed in a three-necked flask reaction apparatus, and the inside thereof was dried to remove water and replaced with a nitrogen or argon atmosphere. The tetrahydrofuran (20 ml) was slowly added to the reaction apparatus and covered with magnesium metal sufficiently. After the reaction mixture was kept in a stable state, 27.9 ml (0.25 millimolar) of dimethylchlorosilane (Me2HSiCl) was slowly added dropwise to the reaction apparatus. This was the first solution. Separately, dibromobenzene, 24.7 g (0.11 mole) and tetrahydrofuran (60 ml), were placed in a feeding tube or a dose pump to be mixed into a dibromobenzene solution, which was the second solution. A small amount (approximately 0.5 ml) of the second solution is slowly added dropwise to the first solution in the reaction device to initiate the Grinnard reaction. The dripping rate was controlled and the feed was slowly fed into the reaction apparatus so that the reaction mixture was maintained in a non-boiling state at a reaction temperature of about 50 to 60C. After the addition was completed, dibromobenzene in the feed tube was rinsed with a small amount of tetrahydrofuran (5 to 10 ml) into the reactor as a third solution, which was heated to reflux for 16 hours. If there is a device temperature sensor, the internal reaction temperature required for the reflux of tetrahydrofuran is 65 C. If the temperature sensor is not installed, the temperature of the oil bath is about 75 C ~ 85 C, depending on the size of the reaction device. After the reaction was completed, distilled water (150 ml) was slowly added dropwise to the third solution in the reaction apparatus to quench the reaction. After continuous stirring for 5 to 10 minutes, two immiscible solutions were obtained, which was the fourth solution. The fourth solution was transferred to a separatory funnel, and the aqueous layer was extracted with ether (60 ml/time, 3 times) to collect the upper organic solvent layer. Separately wash with water (100 ml) and dry saturated brine (120 ml). After drying over anhydrous sodium sulfate followed by filtration, the filtrate was concentrated under reduced pressure to give 20.4 g of the dinonane product of formula (A-1) in 98% yield. |
| 98% |
|
Setup a reaction device, such as a feeding pipe and a reflux pipe, on a three-necked round bottom flask. Put 6.1 g (0.25 mol) of metallic magnesium in the three-necked round bottom flask. Dry the inside of the three-necked round bottom flask. Introduce nitrogen gas or argon gas into the three-necked round bottom flask. Introduce 20 mL of tetrahydrofuran into the reaction device slowly, cover it fully with metallic magnesium, and stir the reactants. Introduce 27.9 mL (0.25 mmol) of dimethylsilyl chloride (Me2HSiCl) into the reaction device slowly, thereby producing the first solution. Producing the second solution entails introducing 24.7 g (0.11 mol) of dibromobenzene and 60 mL of tetrahydrofuran into the feeding pipe or a dosage transmission pump to blend the dibromobenzene solution. Drip 0.5 mL of the second solution slowly to the first solution in the reaction device, so as to trigger the Grignard reaction. Then, feed the mixed first and second solutions to the reaction device slowly enough to prevent the reacting mixture from boiling; meanwhile, the reaction temperature ranges from 50 C. to 60 C. Afterward, residual dibromobenzene in the feeding pipe is washed into the reaction device by 510 mL of tetrahydrofuran to produce the third solution, and then the third solution is heated and refluxed for 16 hours. In the presence of a device temperature sensor, the reflux of tetrahydrofuran requires an internal reaction temperature of 65 C. In the absence of any device temperature sensor, the reflux of tetrahydrofuran requires an oil or sand bath tank temperature of 75 C.85 C., depending on the size of the reaction device. Upon completion of the reaction, 150 mL of distilled water is slowly introduced into the third solution in the reaction device to trigger a quenching reaction, and then the third solution is stirred continuously for 510 minutes to form two layers of immiscible solution known as the fourth solution. Transfer the fourth solution to a separatory funnel to extract the aqueous layer with 60 mL of ether thrice, and then collect the organic supernatant before rinsing it with 100 mL of water and 120 mL of saturated saline solution for the sake of drying. A drying process is performed with anhydrous sodium sulfate, and then filtration is performed so that the filtrate is depressurized and concentrated to obtain 20.4 g of disilane structural formula (A-1), with a yield of 98%. Referring to FIG. 3, it shows the 1H-NMR spectrum for phenylene disilane according to an embodiment of the present invention. |
| 84.7 - 90.5% |
With magnesium; In diethyl ether; toluene; |
[0028] A series of runs was made using a co-solvent comprising diethyl ether and toluene, using procedures similar to those utilized in Example 3, to prepare 1,4-bis(dimethylsilyl)benzene from the reaction of either 1,4-dibromobenzene or 1,4-dichlorobenzene and Me2HSiCl. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping