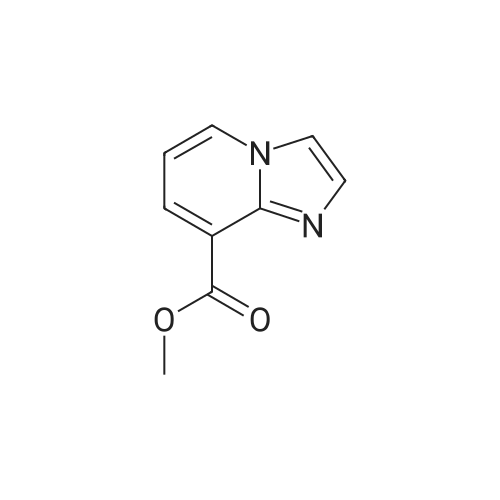
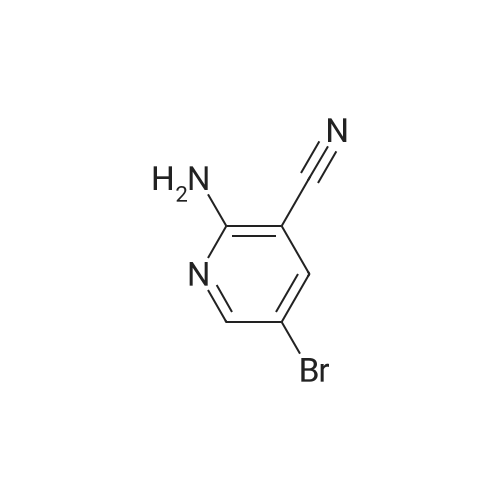
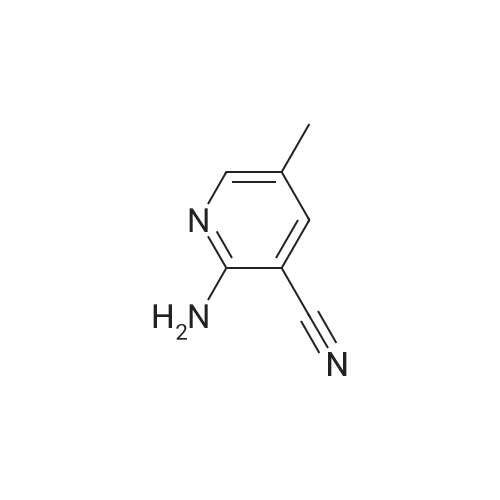
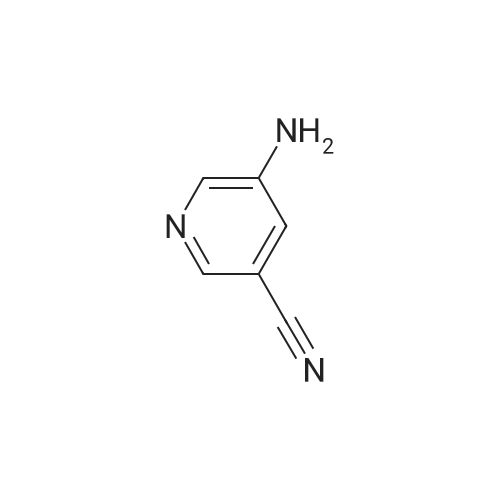
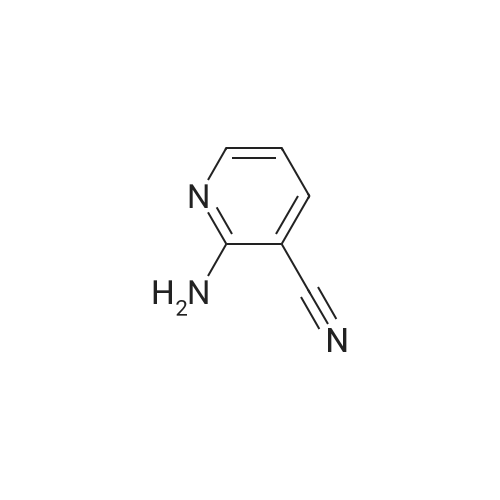
| 96% |
With bromine; sodium carbonate; acetic acid; at 20℃; for 0.833333h; |
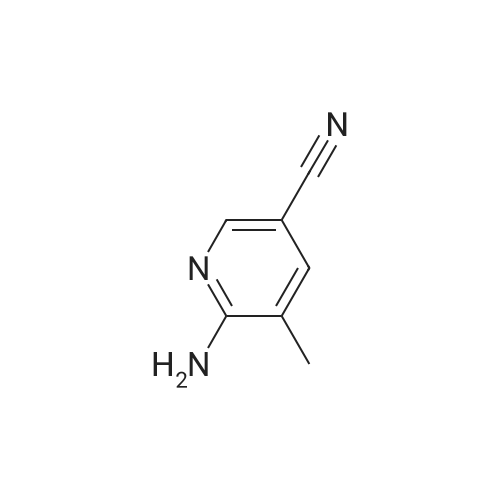
Compound 9: 2-Aminonicotinonitrile 8 (100 g, 0.839 mol) was dissolved in HOAc (800 mL). To the solution was added Na2CO3 (88.97 g, 0.839 mol). Then, Br2 (46.4 mL, 0.923 mol) was added dropwise. The reaction mixture was stirred at room temperature for 50 min. To the mixture was added water (600 mL). The mixture was cooled to about 5 C. The precipitate thus formed was collected by filtration and dried to give compound 9 (207 g, 96%). |
| 94.6% |
With bromine; sodium carbonate; acetic acid; at 20℃; for 18h;Inert atmosphere; |
Step 1: Preparation of 2-amino-5-bromonicotinonitrile (42) 2-Aminonicotinonitrile (95.0 g, 0.80 mol, 1.0 eq) was dissolved in HOAc (2 L) and then Na2CO3 (93.1 g, 0.88 mol, 1.1 eq) was added. Then Br2 (142.6 g, 0.88 mol, 1.1 eq) in HOAc (2 L) was added dropwise to the mixture. The resulting mixture was stirred at room temperature for 18 hours, which was then poured into ice water (10 L), filtered, washed with water (2 L×2) and dried to give the pure intermediate 42 (150.0 g, 0.75 mol, 94.6%). 1H NMR (400 MHz, DMSO-d6) delta 8.28-8.27 (d, J=2.4 Hz, 1H), 8.15-8.14 (d, J=2.4 Hz, 1H), 7.13 (s, 2H). |
| 80% |
With bromine; sodium carbonate; acetic acid; at 20℃; for 1h; |
To a solution of 2-aminonicotinonitrile (1.5 g, 12.5 mmol, 1.0 eq.) in AcOH (30 mL) was added Na2003 (1.3 g, 12.5 mmol). Bromine (0.7 ml, 13.8 mmol) was added drop wise to the resulting suspension and reaction mixture was stirred at rt for 1 h. The orange precipitate formed was collected by filtration, washed with water and dried to afford 2.0 g (80%) of 1-34 as a yellow solid. NMR (400 MHz, DMSO) 6 8.27 (5, 1 H), 8.15 (5, 1 H), 7.12 (brs, 2H). |
| 78% |
With bromine; In acetic acid; at 10 - 20℃; for 22h; |
Bromine (1.1 mL, 21 mmol) in ACOH (3 mL) was added dropwise to a solution of 2-AMINO-NICOTINONITRILE (1.00 g, 8.4 mmol) in ACOH (20 mL) at 10 C. The orange mixture was stirred for 22 hours at ambient temperature then diluted with ether (100 mL). The resultant precipitated salt was filtered, washed with ether and dried on air. The precipitate was suspended in water (100 mL), neutralized with 1N NAOH, filtered, washed with water and dried on air to give 1.29 g (78%) title COMPOUND. LH NMR (300 MHz, DMSO-d6) 8 8.27 (d, J= 2. 5HZ, 1H), 8. 14 (d, J= 2. 5HZ, 1H), 7.13 (s, br, 2H). MS (ESI) INULE : 197.9655 (M+H) +. |
| 73% |
With bromine; sodium carbonate; acetic acid; at 0 - 20℃; for 2h; |
1.1 2-Amino-5-bromo-nicotinonitrile To a solution of 2-amino-nicotinonitrile (0.50 g; 4.11 mmol) in acetic acid (10 mL) was added sodium carbonate (0.48 g; 4.52 mmol) at 0 C followed by the dropwise addition of bromine (0.74 g; 4.52 mmol). The reaction mixture was stirred at ambient temperature for 2 h. The solvent was evaporated under vacuum, the residue was suspended in water (50 mL), filtered by suction and dried to afford the title compound (0.60 g; 73%). The product was used in the next step without further purification; 1H NMR (400 MHz, DMSO-d6) delta 8.26 (d, J = 2.5 Hz, 1 H), 8.14 (d, J = 2.5 Hz, 1H), 7. 3 (brs, 2H); LC/MS (B), Rt: 2.59 min; (M+2H) 200. |
| 69% |
With dihydrogen peroxide; 1-butylpyridinium bromide; toluene-4-sulfonic acid; In 1,2-dimethoxyethane; at 80℃; for 24h;Schlenk technique; Inert atmosphere; Green chemistry; |
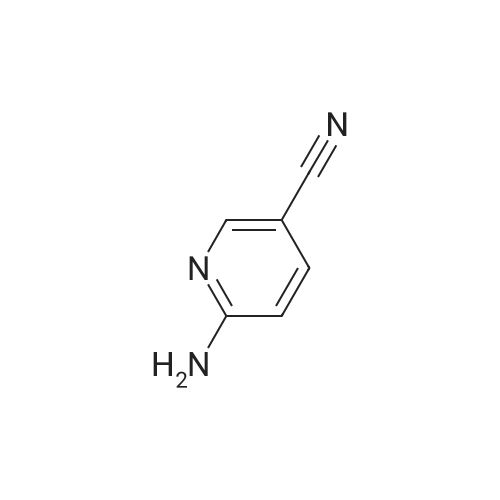
General procedure: To a mixture of 2-aminopyridine (0.5 mmol, 1 equiv), p-TSA (0.4 mmol,0.8 equiv), 1-butylpyridinium bromide (1.5 mmol, 3 equiv) in a 50 mL Schlenk tube were added 1,2-dimethoxyethane (2 mL) under air. Then H2O2 (1.2 mmol, 2.4 equiv) was added. The mixture was stirred at 80C for 24 h. And then the mixture was purified by silica gel column chromatography (petroleum ether/ethyl acetate) to give the products. |
|
With bromine; sodium carbonate; acetic acid; at 20℃; for 2h; |
To a stirred solution of compound 1 (560 mg) in HOAc (14 mL) was added sodium carbonate (487 mg, ) and bromine (808 mg)at room temperature. The reaction mixture was stirred at room temperature for 2h. The resulting solids were collected by filtration and dried in vacuum to give compound 2 |
|
With bromine; sodium carbonate; acetic acid; at 20℃; for 2h; |
Step-1 [0068] To a stirred solution of compound 1 (560 mg) in HOAc (14 mL) was added sodium carbonate (487 mg,) and bromine (808 mg) at room temperature. The reaction mixture was stirred at room temperature for 2 h. The resulting solids were collected by filtration and dried in vacuum to give compound 2 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping