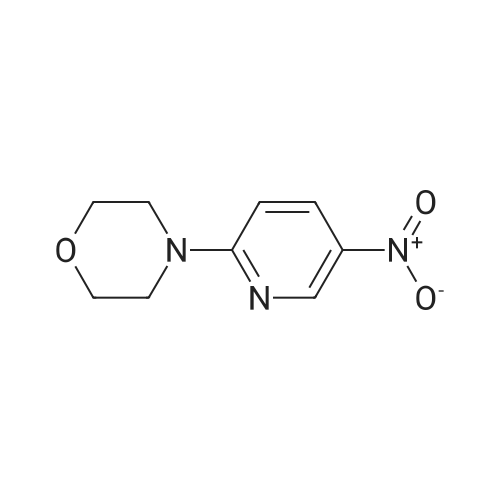
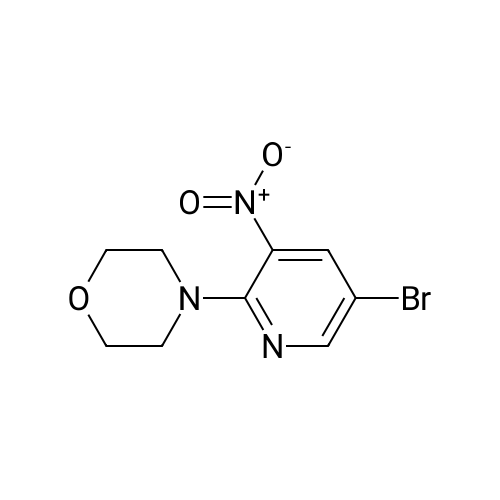
Alternatived Products of [ 24255-27-4 ]
Product Details of [ 24255-27-4 ]
| CAS No. : | 24255-27-4 |
MDL No. : | MFCD00452814 |
| Formula : |
C9H11N3O3
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | QMINJWUSSJFQBR-UHFFFAOYSA-N |
| M.W : |
209.20
|
Pubchem ID : | 2795439 |
| Synonyms : |
|
Application In Synthesis of [ 24255-27-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 24255-27-4 ]
- 1
-
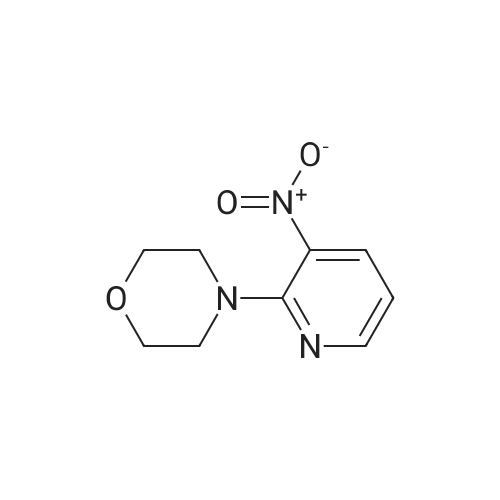 [ 24255-27-4 ]
[ 24255-27-4 ]

-
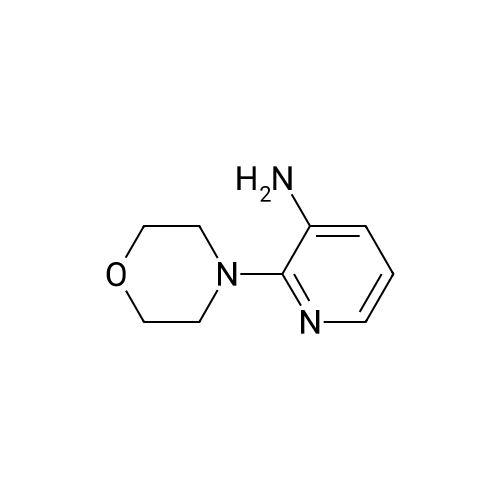 [ 51627-47-5 ]
[ 51627-47-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 90.1% |
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; for 10h; |
4-(3-Nitropyridin-2-yl)morpholine (11.0 g, 52.6 mmol) was dissolved in methanol (100 mL)Add 10% palladium on carbon (5.6 g, 5.3 mmol),Hydrogen was introduced and reacted at room temperature for 10 h.After the reaction is completed, the solvent is distilled off under reduced pressure.The residue was dissolved in water (100 mL).Extracted with ethyl acetate (100 mL×3),The organic phase was collected and dried over anhydrous sodium sulfate.The solid was filtered off, and the solvent was evaporated under reduced pressure.The residue was passed through a silica gel column (mobile phase: PE: EA = 10:1).2-morpholinopyridine-3-amine gray solid 8.5 g was obtained in a yield of 90.1%. |
|
With hydrogenchloride; tin(II) chloride dihdyrate; In ethanol; water; at 80℃; for 5h;Inert atmosphere; |
General procedure: In a 100 mL three neck round bottom flask equipped with a stirring bar and reflux condenser, 2-amino-3-nitropyridine derivative 8a or 8b was dissolved in EtOH (25 mL) and water (25 mL). After stirring for 10 min, SnCl 2 ·2H 2 O (1.5 equiv) and HCl were added, and the reaction mixture was refluxed at 80 C for 5 h. After reaction completion (analysis by TLC), the mixture was allowed to reach room temperature and washed with 1M KOH. The layers were separated and the aqueous layer was further extracted with ethyl acetate (3 X 30 mL). The combined organic phases were washed with brine and dried over anhydrous Na 2 SO 4 , filtered and concentrated under reduced pressure. The crude solid was purified via column chromatography with silica gel to obtain the appropriate 2,3-diaminopyridine derivatives 9a or 9b. |
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; |
At room temperature, <strong>[24255-27-4]4-(3-nitropyridin-2-yl)morpholine</strong> 4A (376 mg, 1.8 mmol) was dissolved in 10 mL MeOH, and 10% Pd/C (100 mg) was added.Stir overnight under hydrogen protection. LCMS showed that the reaction was complete, Pd/C was removed by filtration, and the filtrate was spin-dried to obtain 2-morpholinylpyridin-3-amine 4B as an off-white solid (300 mg, 133%), which was directly used in the next reaction without further purification. |
- 2
-
 [ 110-91-8 ]
[ 110-91-8 ]

-
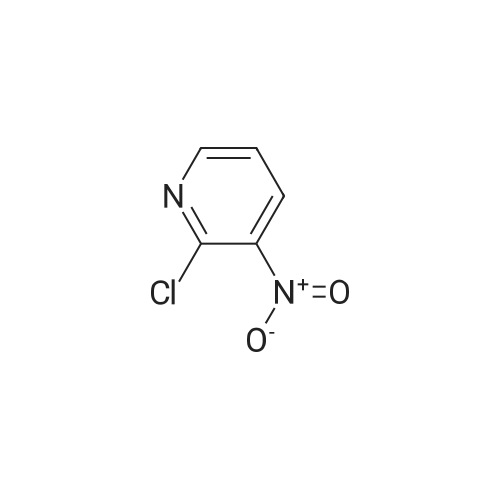 [ 5470-18-8 ]
[ 5470-18-8 ]

-
 [ 24255-27-4 ]
[ 24255-27-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 95% |
With triethylamine; In 1,4-dioxane; for 1h;Reflux; |
At room temperature, dissolve 2-chloro-3-nitropyridine (300mg, 1.89mmol) in 10mL 1,4-dioxane, add TEA (0.78mL, 5.67mmol) and morpholine (181mg, 2.08mmol) , Reflux for 1 hour,LC-MS monitoring showed that the reaction was complete. The reaction solution was concentrated to remove the solvent, and 30 mL of water and 50 mL of ethyl acetate were added to dissolve the residue. The ethyl acetate layer was separated. The aqueous layer was extracted with ethyl acetate (50 mL×2). The organic layers were combined and dried over anhydrous sodium sulfate. Rotate to dryness to obtain 4-(3-nitropyridin-2-yl)morpholine 4A as a yellow solid (376 mg, 95%), which was directly used in the next reaction without further purification. |
| 82.2% |
With potassium carbonate; In N,N-dimethyl-formamide; at 85℃; for 6h; |
2-Chloro-3-nitropyridine (20.0 g, 126.2 mmol) was dissolved in DMF (160 mL)Add potassium carbonate (52.3 g, 378.5 mmol),Slowly added morpholine (22.0 g, 252.3 mmol),The reaction was carried out at 85 C for 6 h. The reaction is completed,Cool to room temperature,Add 200mL of pure water,Extracted with ethyl acetate (200 mL × 2),Collect organic phase,Dry over anhydrous sodium sulfate,Filter out the solids,Evaporate the solvent under reduced pressure.The residue was passed through a silica gel column (mobile phase: PE: EA = 10:1).4-(3-Nitropyridin-2-yl)morpholine yellow solid 21.7 g,yield: 82.2%. |
|
With triethylamine; In tetrahydrofuran; at 65℃; for 2h;Inert atmosphere; |
General procedure: In a 100 mL three neck round bottom flask equipped with a stirring bar and reflux condenser, 2-chloro-3-nitropyridine 5, 1 equiv. of primary or secondary amine, and Et 3 N (1 equiv.) were dissolved in THF (3 mL). The reaction mixture is refluxed at 65 C for 2 h. After the reaction was complete (analyzed by TLC), the mixture was allowed to reach room temperature. The mixture was washed with water (30 mL), and extracted with ethyl acetate (3 X 30 mL). The combined organic phases were washed with brine and dried over anhydrous Na 2 SO 4 , filtered, and concentrated under reduced pressure. The crude oil was purified via column chromatography with silica gel to obtain the appropriate 2-amino-3-nitropyridine derivative 8a or 8b. |
Reference:
[1]International Journal of Chemical Kinetics,1997,vol. 29,p. 599 - 605
[2]International Journal of Chemical Kinetics,1997,vol. 29,p. 599 - 605
[3]Bioorganic and Medicinal Chemistry,2007,vol. 15,p. 1586 - 1605
[4]Patent: CN111848591,2020,A .Location in patent: Paragraph 0272-0275
[5]Journal of Organic Chemistry,2021,vol. 86,p. 8900 - 8925
[6]Patent: CN107840820,2018,A .Location in patent: Paragraph 0016; 0026; 0035; 0038; 0046; 0056; 0066; 0076
[7]Journal of the Iranian Chemical Society,2014,vol. 11,p. 289 - 295
[8]Bioorganic and Medicinal Chemistry,2018,vol. 26,p. 884 - 890
| Yield | Reaction Conditions | Operation in experiment |
|
With triethylamine; In tetrahydrofuran; at 65℃; for 2h; |
General procedure: In a 100 mL three neck round bottom flask equipped with a stirring bar and reflux condenser, 2-chloro-3-nitropyridine 5, 1 equiv. of primary or secondary amine, and Et3N (1 equiv.) were dissolved in THF (3 mL). The reaction mixture is refluxed at 65 C. for 2 h. After the reaction was complete (analyzed by TLC), the mixture was allowed to reach room temperature. The mixture was washed with water (30 mL), and extracted with ethyl acetate (3×30 mL). The combined organic phases were washed with brine and dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude oil was purified via column chromatography with silica gel to obtain the appropriate 2-amino-3-nitropyridine derivative 8a or 8b. |
- 4
-
 [ 24255-27-4 ]
[ 24255-27-4 ]

-
 [ 926009-67-8 ]
[ 926009-67-8 ]
- 5
-
 [ 24255-27-4 ]
[ 24255-27-4 ]

-
 [ 926009-68-9 ]
[ 926009-68-9 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping