|
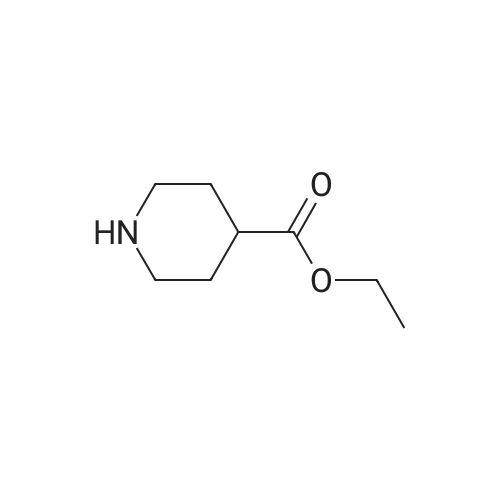
With hydrogenchloride; sodium hydroxide; In ethanol; |
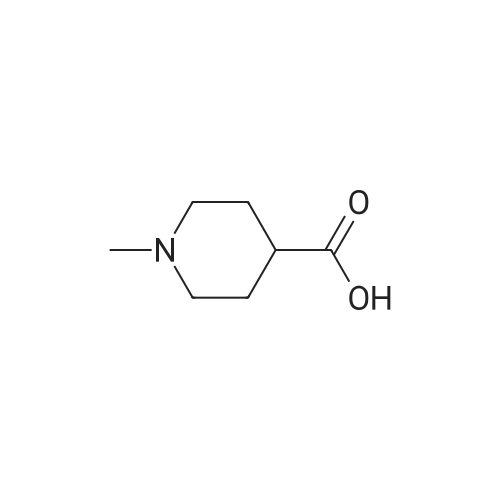
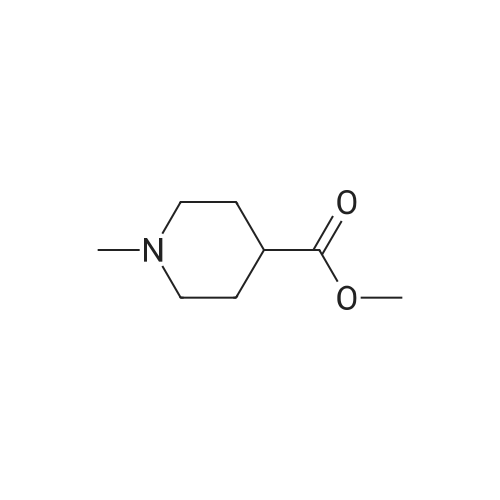
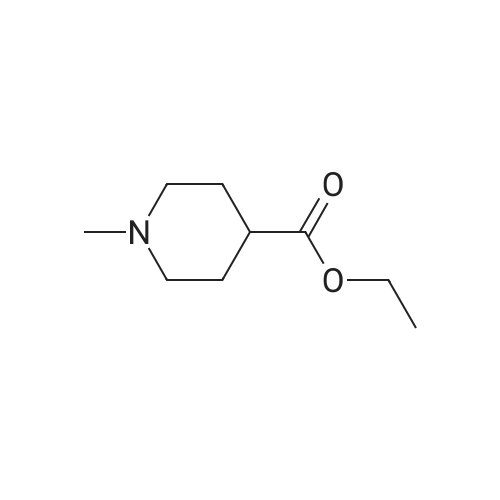
1-Methyl-4-piperidinecarboxylic acid may be prepared in the following manner: 12.5 cm3 of a 4 N aqueous sodium hydroxide solution are added, at 20° C., to 7.60 g of ethyl 1-methyl-4-piperidinecarboxylate in solution in 35 cm3 of ethanol. After stirring for 20 hours, the reaction mixture is concentrated to a reduced volume and then neutralized with 12.5 cm3 of a 4 N aqueous hydrochloric acid solution and finally concentrated to dryness under reduced pressure (2.7 kPa). The residue is stirred in 60 cm3 of anhydrous ethanol, and then filtered. The filtrate is concentrated to dryness under reduced pressure (2.7 Kpa) at 20° C., to give 6.3 g of 1-methyl-4-piperidinecarboxylic acid in the form of a white solid. 1H NMR spectrum (300 MHz, (CD3)2SO d6, delta in ppm): 1.56 (mt: 2H); 1.78 (mt: 2H); 1.98 (dt, J=11.5 and 2.5 Hz: 2H); 2.13 (mt: 1H); 2.18 (s: 3H); 2.74 (broad d, J=11.5 Hz: 2H). |
|
With sodium hydroxide; water; at 15 - 30℃; |
A mixture of ethyl piperidine-4-carboxylate (4.72 g), methyl iodide (2.24 mL), potassium carbonate (8.29 g) and acetonitrile (50 mL) was stirred at room temperature for 2 hrs. The reaction solution was concentrated under reduced pressure, and water (150 mL) was added thereto, followed by extracting with ethyl acetate (150 mL). The ethyl acetate layer was washed with saturated brine (100 mL), and dried over anhydrous magnesium sulfate, then concentrated under reduced pressure. To the residue (2.64 g) was added 1 N aqueous solution of sodium hydroxide (20 mL), and stirred at room temperature overnight. To the reaction solution was added 1 N hydrochloric acid (20 mL) to neutralize, and concentrated under reduced pressure. To the residue was added ethanol, and the precipitate was filtered off, and the filtrate was concentrated under reduced pressure. After repeating this operation again, to the residue were added ethanol and ethyl acetate to crystallize, which gave 1-methylpiperidine-4-carboxylic acid as colorless solid (1.79 g). 1H-NMR (CD3OD) : 1. 80-1.98 (2H,m), 2.00-2.14 (2H,m), 2.28-2.42 (1H,m), 2.78(3H,s), 2.88-3.04(2H.m), 3.32-3.44(2H.m). |
|
|
A mixture of ethyl piperidine-4-carboxylate (4.72 g), methyl iodide (2.24 mL), potassium carbonate (8.29 g) and acetonitrile (50 mL) was stirred at room temperature for 2 hrs. The reaction mixture was concentrated under reduced pressure and water (150 mL) was added. The mixture was extracted with ethyl acetate (150 mL). The ethyl acetate layer was washed with saturated brine (100 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. A 1N aqueous sodium hydroxide solution (20 mL) was added to the residue (2.64 g), and the mixture was stirred overnight at room temperature. The reaction mixture was neutralized by adding 1N hydrochloric acid (20 mL) and the mixture was concentrated under reduced pressure. Ethanol was added to the residue, and the precipitate was filtered off. The filtrate was concentrated under reduced pressure. This step was repeated and ethanol and ethyl acetate were added to the residue for crystallization to give 1-methylpiperidine-4-carboxylic acid (1.79 g) as a colorless solid.1H-NMR (CD3OD) : 1.80-1.98 (2H,m), 2.00-2.14 (2H,m), 2.28-2.42 (1H,m), 2.78(3H,s), 2.88-3.04(2H.m), 3.32-3.44(2H.m). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping